Polyclonal Antibody Production
Firstly, your project is discussed with our team, we help you to make the right decisions and personalize the process according to your goals. For the production of polyclonal antibodies, we select New Zealand white rabbits. Additionally, we offer the development of polyclonal antibodies in mice and rats.
We can generate policlonal antibodies towards a variety of antigens including small molecules, peptides, proteins, and cells.
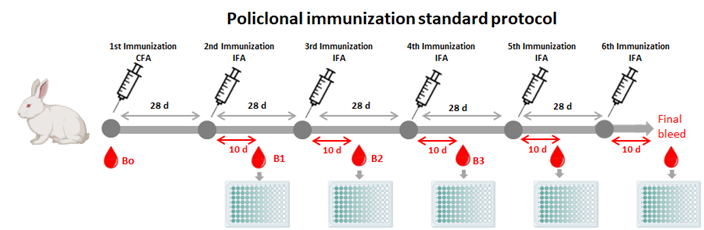
Our standard immunization protocol includes monthly inoculations with Freund’s adjuvant over a 6-month period. To assess immune response progress, blood samples are taken 10 days after the second inoculation to obtain serum and determine antibody titers using ELISA. Preimmune serum is collected before the first injection for controls. At the end of the process, complete blood collection from the animal is performed surgically under anesthesia, resulting in 50-70 mL of hyperimmune serum per animal. The service provides 5 mL of bleed serum from each test for screening in customer labs.
We can follow your personalized immunization program in addition to our well-established ones.
Customer benefits
- Scientific Consultation: We provide thorough consultation before the project begins.
- Customized Project Proposals: We tailor project proposals to meet your specific requirements.
- Flexible Workflow: Adjustments can be made to the workflow based on results and your specific requests.
Target customer
Organizations involved in research and development, particularly those seeking reliable and customized polyclonal antibody development services, will find our offering essential for their scientific endeavors.
Additional information
Selected references:
- E. Montagut, J. Raya, M.-T. Martín Gómez, L. Vilaplana, B. Rodríguez-Urretavizcaya, M.-P. Marco. An Immunochemical Approach to detect the Quorum Sensing-Regulated Virulence Factor 2-Heptyl-4-Quinoline N-Oxide (HQNO) produced by Pseudomonas aeruginosa Clinical Isolates. Microbiol. Spect., 10(4), 1-12, 2022
- E. Montagut, G. Acosta, F. Albericio, M. Royo, G. Godoy-Tena, A. Lacoma, C. Prat, J.-P. Salvador, M.-P. Marco. Direct Quantitative Immunochemical Analysis of the Autoinducer Peptide IV (AIP-IV) for Diagnosing and Stratifying Staphylococcus aureus infections. ACS Infect. Dis., 8(3), 645-656, 2022.
- G. Colom, J.-P. Salvador, G. Acosta, M. Royo, M.-P. Marco. Competitive ELISA for N-Terminal pro-Brain Natriuretic Peptide (NT-proBNP) determination in human plasma. Analyst, 145, 6719-6727, 2020.








