Design & Development of lipid nanoparticles
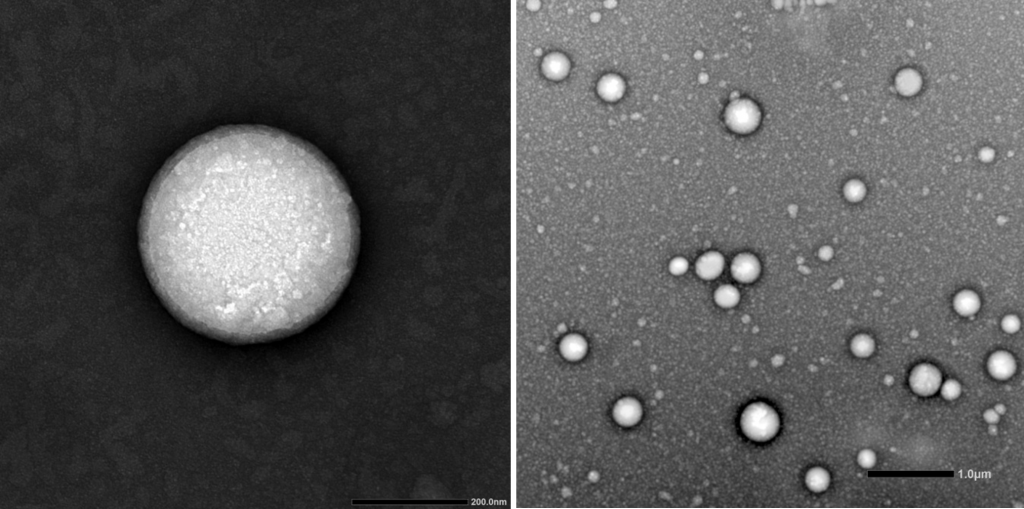
Design & Development of lipid nanoparticles to encapsulate therapeutic actives for drug delivery or gene delivery purposes: SLNs (solid lipid nanoparticles), NLCs (nanostructured lipid carriers), LNPs (self-assembled lipid nanoparticles by microfluidic technology) and niosomes.
Customer benefits
Customized elaboration of nanovehicles to deliver the therapeutic material of interest (mRNA, plasmid DNA, drugs, growth factors, peptides, antibodies…). We are specialists in designing and developing formulations for several therapeutic purposes. Among them, we develop self-assembled lipid nanoparticles, which are formed by fast microfluidic mixing in a NanoAssemblr® platform. These kinds of nanoparticles have emerged as an ideal nanotechnology approach for drug delivery and non-viral gene therapy by the high efficient encapsulation and protection of the therapeutic material from degradation. This microfluidic technology, that has been employed for COVID-19 vaccine, works under GMP conditions and enables reproducibility and scalability, which enhances its clinical translation.
Physicochemical characterization of nanovehicles, following SOPs, regarding mean particle size, polydispersity index and zeta potential. Additional parameters can be determined, such as, pH of nanoparticle suspension, microscopic morphology of the nanoparticles, encapsulation efficiency of the active principle and in vitro release profile. Optionally, biological evaluation can be performed, such as transfection efficiency analysis of non-viral nanovehicles and/or cytotoxicity assays following SOPs that include the ISO 10993-5-2019 Biological evaluation of medical devices.
Target customer
- Customers with a specific target for therapeutic purposes.
- Preclinical use for validation in in vitro and in vivo models.
References
- Gallego I, Villate-Beitia I, Soto-Sánchez C, Menéndez M, Grijalvo S, Eritja R, Martínez-Navarrete G, Humphreys L, López-Méndez T, Puras G, Fernández E, Pedraz JL. Brain Angiogenesis Induced by Nonviral Gene Therapy with Potential Therapeutic Benefits for Central Nervous System Diseases. Mol Pharm. 2020 Jun 1;17(6):1848-1858. doi: 10.1021/acs.molpharmaceut.9b01213.
- Pastor M, Moreno-Sastre M, Esquisabel A, Sans E, Viñas M, Bachiller D, Asensio VJ, Pozo AD, Gainza E, Pedraz JL. Sodium colistimethate loaded lipid nanocarriers for the treatment of Pseudomonas aeruginosa infections associated with cystic fibrosis. Int J Pharm. 2014 Dec 30;477(1-2):485-94. doi: 10.1016/j.ijpharm.2014.10.048.
- Moreno-Sastre M, Pastor M, Esquisabel A, Sans E, Viñas M, Fleischer A, Palomino E, Bachiller D, Pedraz JL. Pulmonary delivery of tobramycin-loaded nanostructured lipid carriers for Pseudomonas aeruginosa infections associated with cystic fibrosis. Int J Pharm. 2016 Feb 10;498(1-2):263-73. doi: 10.1016/j.ijpharm.2015.12.028.
Additional information