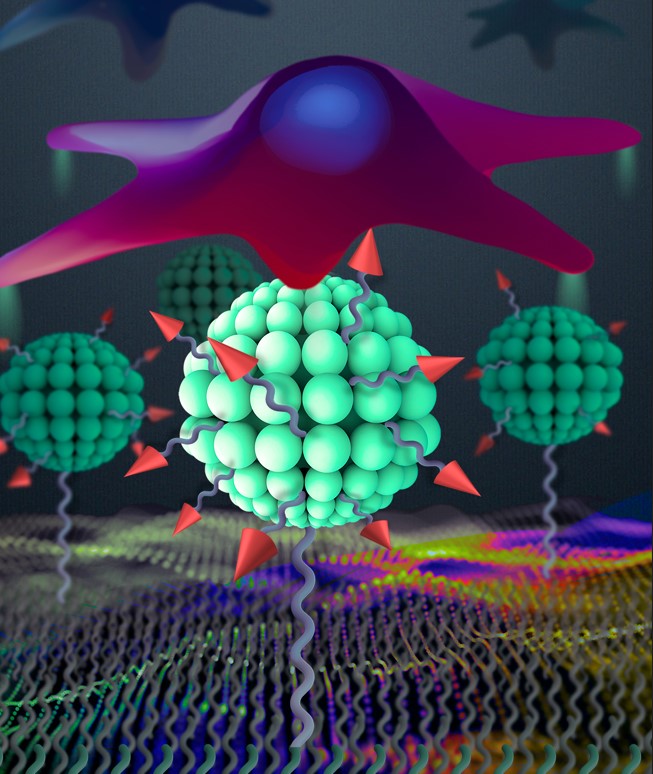
New nanoarchitectonics of RGD peptide developed using quatsomes as robust tool in tissue engineering.
Researchers of three groups of CIBER-BBN at CSIC and IBEC, have created a versatile platform based on hierarchically nanostructured RGD peptide using quatsomes, with proved enhanced cell adhesion. These findings, which arose within the framework of the intramural project of CIBER-BBN “Molecular Biointerfaces for cell guidance” (DynaMo4Vasc), open new possibilities for tissue engineering.
The participation of two NANBIOSIS units were acknowledged in the publication of the research results: the synthesis of RGD derivatives were performed at NANBIOSIS U3 “Synthesis of peptides unit” of CIBER-BBN at IQAC−CSIC. And the design and characterization of quatsomes were done at U6 of NANBIOSIS “Biomaterial Processing and Nanostructuring Unit” of CIBER-BBN at ICMAB-CSIC.
Tissue Engineering and cell adhesion
Tissue engineering pursues the development of functional and easy biological substitutes that allow restoring damaged organ or tissue or maintaining their normal function. The newer approach is the combination of appropriate cells and growth factors with a scaffold that supports the tissue or organ.
The scaffold is crucial since it must provide the conditions and the environment for the adequate cell regulation (adhesion, migration, proliferation, and differentiation), as well as the adequate delivery of bioactive factors (growth and adhesion), so that cells form the new tissue with its proper structure and function.
Cell adhesion (the interaction of the cells with its surroundings) is an important phenomenon for the development of appropriate scaffolds for tissue engineering, as it can ultimately determine cell fate. Thus, the study of the factors that govern cell adhesion and its optimization is essential.
The tripeptide Arg-Gly-Asp (RGD) is the most common peptide responsible for cell adhesion. Although the studies of its surface density and spacing at nanoscale have already shown a significant influence on cell adhesion, the impact of its hierarchical nanostructure is still unexplored.
Quatsomes
Quatsomes are non-liposomal nanovesicles which have been shown to be very homogeneous and stable in different media and which can be easily tuned with a wide range of chemical functionalities.
The Nanomol Group (from CIBER-BBN and ICMAB-CSIC) has been working during the last years with these nanoparticles showing their suitability for applications in nanomedicine, (as nanocarriers and nanocontainers to encapsulate drugs and protein cargoes, or as fluorescent dyes for therapy and diagnostics). This expertise led the researcher to explore the integration of quatsomes with relevant molecules and their use once anchored to a surface.
RGD Nanoarchitecnonics
Nanoarchitectonics is a novel concept that refers to multicomponent systems organized through the supramolecular union of nanometer structures where the main players are not the individual nanoparticles but their interactions, giving rise to new functionalities.
The team of researchers developed a versatile platform based on quatsomes as an effective nanoscopic building block to achieve hierarchical nanostructures of the RGD peptide which were further anchored to gold substrate. In comparison with substrates featuring a homogeneous distribution of RGD peptides, the resulting hierarchical nanoarchitectonic surfaces dramatically enhanced cell adhesion.
These findings open many possible pathways for the understanding of cell behaviour and improve the performance of clinical applications like implants and tissue engineering.
Article of reference:
Marc Martínez-Miguel, Miquel Castellote-Borrell, Mariana Köber, Adriana R. Kyvik, Judit Tomsen-Melero, Guillem Vargas-Nadal, Jose Muñoz, Daniel Pulido, Edgar Cristóbal-Lecina, Solène Passemard, Miriam Royo, Marta Mas-Torrent, Jaume Veciana, Marina I. Giannotti, Judith Guasch, Nora Ventosa, and Imma Ratera. “Hierarchical Quatsome-RGD Nanoarchitectonic Surfaces for Enhanced Integrin-Mediated Cell Adhesion” ACS Appl. Mater. Interfaces 2022, 14, 42, 48179–48193 Publication Date:October 17, 2022 https://doi.org/10.1021/acsami.2c10497