3D Printing and Bioprinting for Pharmaceutical and Medical Applications
Researchers of CIBER-BBN group NanoBioCell – NANBIOSIS U10 Drug Formulation unit Jose Luis Pedraz Muñoz, Laura Saenz del Burgo Martínez, Gustavo Puras Ochoa, Jon Zarate Sesma have edited the book “3D Printing and Bioprinting for Pharmaceutical and Medical Applications”
The increasing availability and decreasing costs of 3D printing and bioprinting technologies are expanding opportunities to meet medical needs. 3D Printing and Bioprinting for Pharmaceutical and Medical Applications discusses emerging approaches related to these game-changer technologies in such areas as drug development, medical devices, and bioreactors.
Key Features:
- Offers an overview of applications, the market, and regulatory analysis
- Analyzes market research of 3D printing and bioprinting technologies
- Reviews 3D printing of novel pharmaceutical dosage forms for personalized therapies and for medical devices, as well as the benefits of 3D printing for training purposes
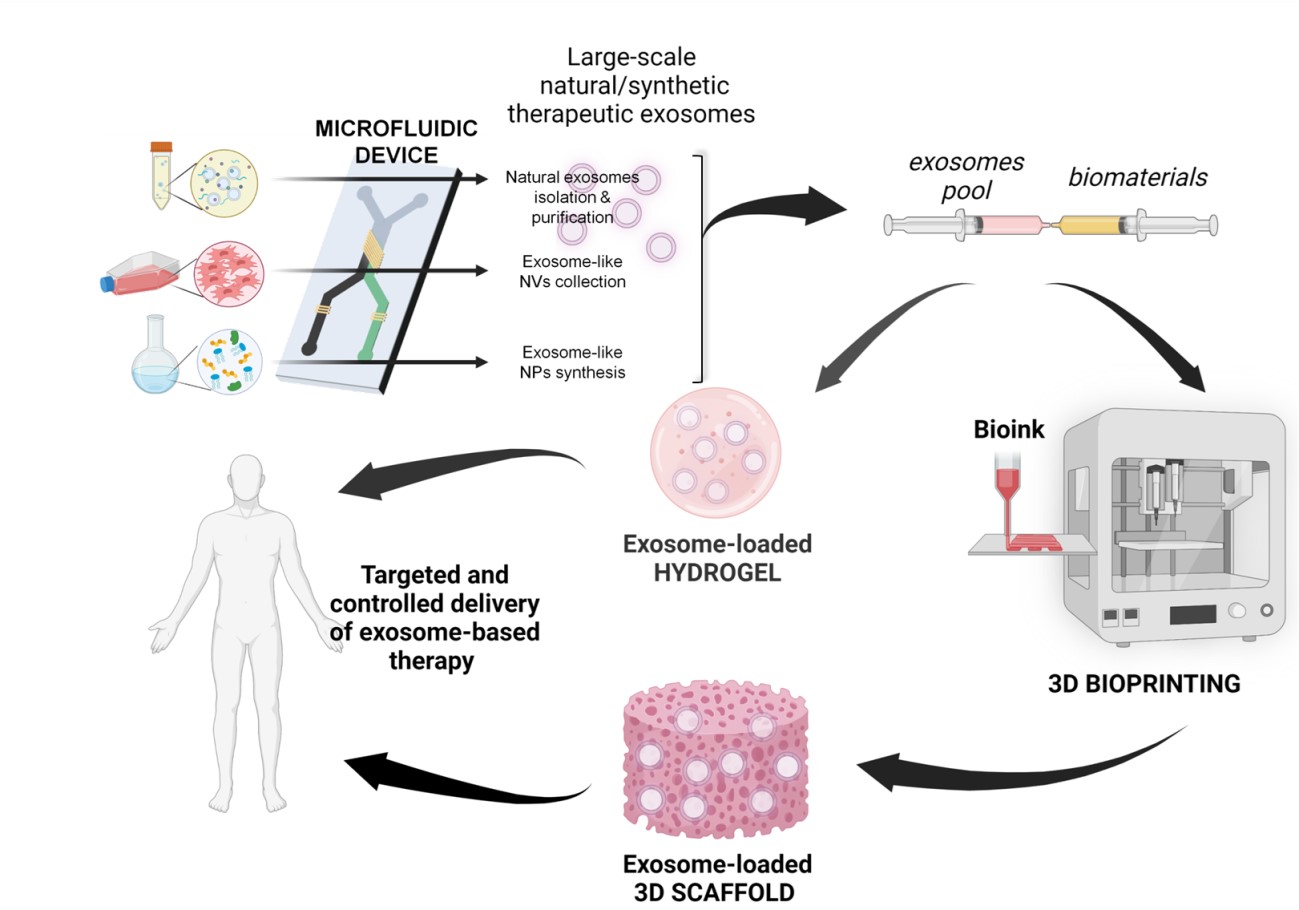
- Covers 3D bioprinting technology, including the design of polymers and decellularized matrices for bio-inks development, elaboration of 3D models for drug evaluation, and 3D bioprinting for musculoskeletal, cardiovascular, central nervous system, ocular, and skin applications
- Provides risk-benefit analysis of each application
- Highlights bioreactors, regulatory aspects, frontiers, and challenges
This book serves as an ideal reference for students, researchers, and professionals in materials science, bioengineering, the medical industry, and healthcare.
For table of contents and further deatails: