Scientists of Units 1 and 18 of NANBIOSIS are coathors of the article “Switching cell penetrating and CXCR4-binding activities of nanoscale-organized arginine-rich peptides” published by Nanomedicine: Nanotechnology, Biology and Medicine
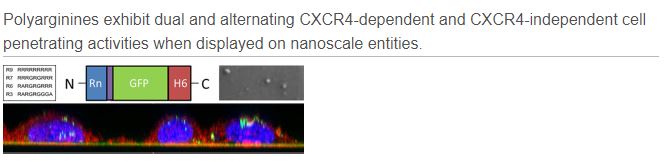
Arginine-rich protein motifs have been described as potent cell-penetrating peptides (CPPs) but also as rather specific ligands of the cell surface chemokine receptor CXCR4, involved in the infection by the human immunodeficiency virus (HIV).
Polyarginines are commonly used to functionalize nanoscale vehicles for gene therapy and drug delivery, aimed to enhance cell penetrability of the therapeutic cargo. However, under which conditions these peptides do act as either unspecific or specific ligands is unknown. The authors have here explored the cell penetrability of differently charged polyarginines in two alternative presentations, namely as unassembled fusion proteins or assembled in multimeric protein nanoparticles. By this, they have observed that arginine-rich peptides switch between receptor-mediated and receptor-independent mechanisms of cell penetration. The relative weight of these activities is determined by the electrostatic charge of the construct and the oligomerizationstatus of the nanoscale material, both regulatable by conventional protein engineering approaches
Protein production has been partially performed by the ICTS “NANBIOSIS”, more specifically by the U1. Protein Production Platform (PPP), whereas the in vivo biodistribution assays were performed in the NANBIOSIS U18. Nanotoxicology Unit,
Article of reference:








