U10. Drug Formulation


U10. Drug Formulation
-
- Scientific Director: Prof. José Luis Pedraz joseluis.pedraz@ehu.es
- Scientific Coordinator: Dr. Idoia Gallego Garrido idoiagallego@hotmail.com
- Entities: Universidad del País Vasco (UPV/EHU)
- Address: Pharmacy & Pharmac. Technology Lab.; Facultad de Farmacia, C/Paseo de la Universidad 7, 01006 Vitoria-Gasteiz (Spain)
- Phone: +34 653593042
- Fax: +34 945 013040
- Web: NANOBIOCEL UPV/EHU
 |
Description
Located at the Faculty of Pharmacy, University of the Basque Country (UPV/EHU), Campus of Alava in Vitoria. The Unit is led by Prof. José Luis Pedraz and consists of large laboratories for cell culture, chromatography equipment, sample preparation and characterization, and one specific for scale preparation of pharmaceutical formulations. Recently the group has incorporated 3D bioprinters of the last generation with different technologies based on extrusion, inkjet, among others. It has also incorporated self-assembly equipment of nanoparticles based on microfluidic technologies.
This Unit can design and evaluate dosage forms both classical and new dosage forms of biotech drugs, DNA, RNA, and vaccines using different methodologies based on micro and nano-medicine and the latter technology based on the microencapsulation of cells, peptides, proteins, and in general of biotech products, as well as the development and design of non-viral vectors for gene therapy, is one of the biggest singularities of this Unit. It counts on the most advanced equipment for micro and nanoencapsulation.
The Unit aims to determine experimentally all the variables needed to develop an optimal formulation and work instructions for preparing final pharmaceutical products.
The pharmaceutical technology applied to drug development involves the selection of materials and procedures that can be adapted to different processes that lead to specific pharmaceutical forms. To do that, the Unit10 counts with the most advanced equipment to cover the development for all the steps of the process.
One of the singularities of this Units is that is GLP certified by the Spanish Medicament Agency
News U10
28 Feb

Advancing new treatments for Retinitis Pigmentosa within NANBIOSIS
NANBIOSIS Unit 10 develops lipid-based nanoparticles for gene and drug delivery, aiming for cost-effective, non-invasive treatments for retinitis pigmentosa. Vitoria, february 2025. Retinitis pigmentosa (RP) is a congenital rare disease that leads to progressive and irreversible vision loss. Both genetic mutations and inflammation contribute significantly to the progression of the disease. Researchers from the Drug [...]
11 Sep

Gene Therapy for Cystic Fibrosis: new inspiring scientific collaboration
Researchers are advancing gene therapies for cystic fibrosis using non-viral delivery methods, focusing on patient needs and innovative treatments. Basque Country, September, 2024 – As the world comes together to mark World Cystic Fibrosis Day, from NANBIOSIS we want to highlight the collaborative efforts between our Unit 10 “Drug Formulation” (U10), the University of the [...]
09 Sep

New gene therapy for Cystic Fibrosis: an interview with Lucía Enríquez
Vasque Country, September, 2024 – In this interview, Lucía Enríquez, a PhD researcher at NANBIOSIS Unit 10, discusses her work on gene therapies for cystic fibrosis, a genetic disease that mainly affects the lungs. Her research focuses on using non-viral vectors to deliver gene-editing tools, like Prime Editing, a variation of CRISPR-Cas9, to correct mutations [...]
27 Jun

Prof. Jose Luis Pedraz, new “Académico de Número” of the Spanish Royal National Academy of Pharmacy
Madrid, June, 2024 – In a distinguished ceremony held at the Spanish Royal National Academy of Pharmacy (Real Academia Nacional de Farmacia), Professor Jose Luis Pedraz Muñoz, a prominent figure at the University of the Basque Country, was officially inducted as an “Académico de Número”. The highest position within the Royal National Academy of Pharmacy. [...]
13 Nov

Rosa Hernández of the NanoBioCel group-NANBIOSIS U10 has received the Female Talent Award 2023 Women and Science
On Thursday, November 2, the Female Talent Award, in the category of Women and Science, was awarded to Rosa Hernández from the NanoBioCel group of CIBER BBN and the U10 Drug Formulation Unit. The award is given by AMPEA (Association of Professional and Business Women of Alava). In her award speech, Rosa Hernández, highlighted that [...]
08 Nov

Impactful research with NANBIOSIS participation in the Poster Tour of CIBER-BBN & CIBEREHD Annual Conference.
2023 CIBER-BBN Annual meeting has taken place at Santemar Hotel, in Santander during November 6-7. This year the format of our annual conferences has been changed towards a collective event scheme between the CIBER-BBN and CIBEREHD thematic areas. On Monday 6 the scientific sessions werecommon for EHD and BBN, with appealing contents for the mixed [...]
15 Oct

Developing Support Technologies for adressing traslational gaps in regenerative medicine.
On the 20th of October, CIBER-BBN group NanoBioCell – NANBIOSIS U10 Drug Formulation unit organises the Conference “Developping support technologies for adressing traslational gaps in regenerative medicine” by Dr. James-J Yoo. The Conference will take place in the Assembly hall of the Faculty of Pharmacy on Friday 20th of October, 2023 from 12:00 to 13:00, [...]
02 Oct

3D Printing and Bioprinting for Pharmaceutical and Medical Applications
Researchers of CIBER-BBN group NanoBioCell – NANBIOSIS U10 Drug Formulation unit Jose Luis Pedraz Muñoz, Laura Saenz del Burgo Martínez, Gustavo Puras Ochoa, Jon Zarate Sesma have edited the book “3D Printing and Bioprinting for Pharmaceutical and Medical Applications” The increasing availability and decreasing costs of 3D printing and bioprinting technologies are expanding opportunities to meet medical needs. 3D [...]
22 Jun
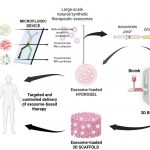
The role of microfluidics and 3D-bioprinting in the future of exosome therapy. A high impact review
Researchers of the NanoBioCel research group of the University of the Basque Country (UPV/EHU) and CIBER BBN, belonging to NANBIOSIS U10 Drug Formulation, Bioaraba, and CONICET Foundation of Argentina, have collaborated in a studio entitled: “The role of microfluidics and 3D-bioprinting in the future of the exosome therapy” which has been published in the journal [...]
23 Nov

Three articles acknoledging NANBIOSIS contribution awarded at the Bioaraba Research and Innovation Conference
Researchers of NANBIOSIS U10 “Drug Formulation” – NanoBiocel research group from CIBER-BBN and UPV/EHU receives 3 awards in the category of Best International Articles at the annual Bioaraba Research and Innovation Conference. On 4 November, the Bioaraba Health Research Institute held its XXIII Research and Innovation Conference in Vitoria. This important annual forum held in [...]