Vall d’Hebron develops magnetic nanoparticle hyperthermia to enhance pancreatic cancer treatment, now advancing to clinical trials.
Barcelona, january 2025. A clinical trial targeting patients with locally advanced pancreatic cancer has been approved following a study led by the Vall d’Hebron Research Institute (VHIR).
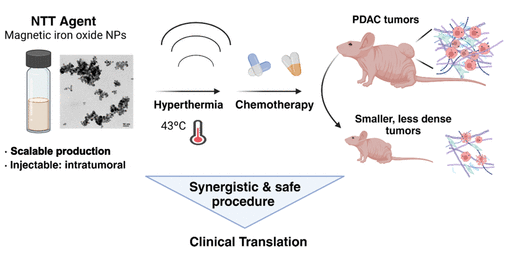
A preclinical study led by the Clinical Biochemistry, Drug Targeting, and Therapy (CB-DDT) group at Vall d’Hebron Research Institute (VHIR), which Unit 20 of NANBIOSIS is integrated, has proposed the use of magnetic nanoparticles and hyperthermia to enhance the treatment of pancreatic adenocarcinoma. The goal is to penetrate the desmoplastic stroma—the dense tissue surrounding these tumors—which acts as a barrier to chemotherapy. Overcoming this matrix to directly reach the tumor is crucial for improving the survival rate of pancreatic cancer patients, which currently stands at only 16% at five years.
The research, conducted in collaboration with CIBER-BBN, one of the nodes of NANBIOSIS, and other national and international research centers, has been published in Applied Materials & Interfaces. Based on these promising results, a clinical trial led by the Vall d’Hebron Institute of Oncology (VHIO) has been initiated to assess this approach in patients with locally advanced pancreatic cancer.
A Multidisciplinary Approach in the NoCanTher Project
This investigation is part of the NoCanTher project, which brings together experts from eleven national and international institutions. Funded by the Horizon 2020 program, the NoCanTher consortium seeks innovative strategies against pancreatic adenocarcinoma by leveraging magnetic nanoparticles. It is estimated that 20% of pancreatic cancer patients have this specific pathology, characterized by tumors without metastasis but which cannot be surgically removed. Currently, the only available treatment option is palliative chemotherapy.
The project focuses on developing iron-based magnetic nanoparticles that, when exposed to an alternating magnetic field, generate heat (magnetic hyperthermia). This heat can be used to make the desmoplastic stroma more permeable, allowing chemotherapy to reach the malignant cells more effectively. The treatment’s efficacy is enhanced to the point where tumor cells can be destroyed.
Promising Results and Clinical Implications
The study demonstrates that when these nanoparticles are injected directly into the tumor, the hyperthermia they generate reduces tumor volume and induces physical changes that facilitate chemotherapy penetration. “This highlights a significant synergistic effect between nanoparticle-induced hyperthermia and chemotherapy in treating pancreatic cancer,” explains Dr. Simón Schwartz Jr, Director of Research and Innovation at the Department of Biochemistry and co-principal investigator of the project alongside Dr. Ibane Abasolo, currently a principal researcher at the Institute of Advanced Chemistry of Catalonia and the Scientific Director of Unit 20.
“This highlights a significant synergistic effect between nanoparticle-induced hyperthermia and chemotherapy in treating pancreatic cancer”
Dr. Simón Schwartz Jr
Beyond evaluating the treatment’s efficacy in humans, the researchers will also collect blood samples from trial participants to determine whether this therapy reduces the number of circulating tumor cells in the bloodstream, particularly cancer stem cells, which are responsible for generating new cancer cells and metastasizing. Although this remains a relatively new field of research, for cases where external beam radiotherapy poses a higher risk of toxicity, this innovative approach could offer a viable treatment alternative—especially for patients who do not respond to standard therapies.
More information about the publication can be found here.
What is NANBIOSIS?
The goal of NANBIOSIS is to provide comprehensive and integrated advanced solutions for companies and research institutions in biomedical applications. All of this is done through a single-entry point, involving the design and production of biomaterials, nanomaterials, and their nanoconjugates. This includes their characterization from physical-chemical, functional, toxicological, and biological perspectives (preclinical validation).
Leading scientists
The main value of NANBIOSIS is our highly qualified and experienced academic scientists, working in public institutions, renowned universities and other research institutes.
Custom solutions
Designed for either scientific collaboration or the private industry, we adapt our services to your needs, filling the gaps and paving the way towards the next breakthrough.

Cutting-Edge facilities
Publicly funded, with the most advanced equipment, offering a wide variety of services from synthesis of nanoparticles and medical devices, including up to preclinical trials.
Standards of quality
Our services have standards of quality required in the pharmaceutical, biotech and medtech sectors, from Good Practices to ISO certifications.
In order to access our Cutting-Edge Biomedical Solutions with priority access, enter our Competitive Call here.
NANBIOSIS has worked with pharmaceutical companies of all sizes in the areas of drug delivery, biomaterials and regenerative medicine. Here are a few of them:









