
A new TPU surface modification with an antimicrobial protein prevents biofilm formation in implants, offering an alternative to antibiotics and metal coatings.
Barcelona, february 2025. Infections associated with medical implants are a serious health issue. A new study presents a chemical modification of thermoplastic polyurethane (TPU) with an antimicrobial protein that reduces the formation of biofilms of multidrug-resistant bacteria, offering an alternative to antibiotics and metal coatings in these devices.
A team of researchers has developed a new strategy to chemically modify TPU, a material widely used in medical devices, to endow it with antibacterial properties and prevent infections associated with biomedical implants. This innovation could represent a significant advancement in the safety and durability of medical implants. The study is part of a project funded by La Marató de TV3.
The study, led by Imma Ratera, researcher at the Nanomol-Bio group at ICMAB-CSIC and CIBER-BBN, and recently published in ACS Applied Bio Materials, describes how the chemical modification of the TPU surface and the self-assembled monolayer strategy are key to enabling the anchoring of the recombinant human α-defensin 5 (HD5) protein.
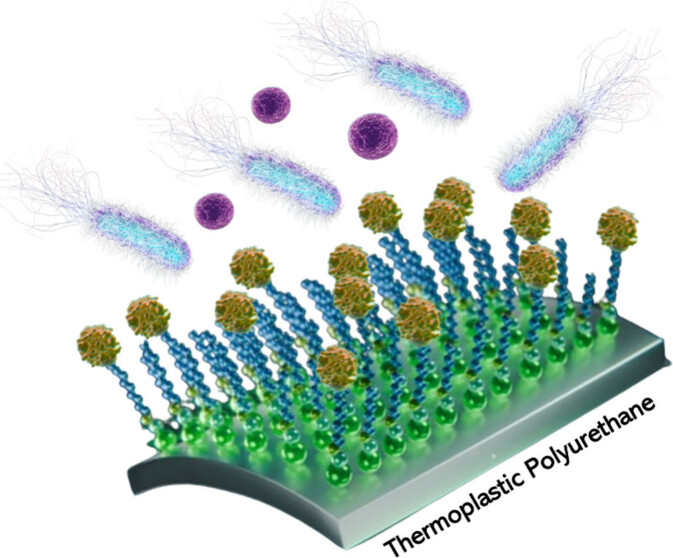
This specific surface functionalization promotes interaction with the antimicrobial protein, effectively inhibiting bacterial biofilm formation. The surface modification was achieved through a three-step process: activation of TPU with hexamethylene diisocyanate (HDI), interfacial reaction with polyethylene glycol (PEG) derivatives, and finally, a simple click reaction between the PEG-maleimide terminated assembled monolayer and the HD5 protein.
The material has been characterized using advanced surface science techniques, confirming its antibacterial efficacy. The results show a significant reduction in the formation of biofilms of resistant gram-positive and gram-negative bacteria, such as Pseudomonas aeruginosa, methicillin-resistant Staphylococcus aureus (MRSA), and methicillin-resistant Staphylococcus epidermidis (MRSE).
This technology offers a promising alternative to antibiotics and metals like silver, addressing the issue of antimicrobial resistance in implantable medical devices. “This breakthrough could represent a paradigm shift in preventing infections in medical implants, reducing complications, and improving patient safety,” says Ratera.
This discovery opens new avenues for the development of antimicrobial surfaces in medical devices, with great potential to improve clinical outcomes and reduce healthcare costs associated with hospital-acquired infections.
The study was conducted by researchers from the Institute of Materials Science of Barcelona (ICMAB-CSIC) and the Biomedical Research Networking Center – Bioengineering, Biomaterials, and Nanomedicine (CIBER-BBN), in collaboration with the Institute of Agrifood Research and Technology (IRTA), the Hospital Clínic-Institute of Biomedical Research August Pi i Sunyer (IDIBAPS) with the Biomedical Research Networking Center on Infectious Diseases (CIBER-INFEC), and the Hospital Universitari Parc Taulí.
The luminescence characterization of the modified TPU surfaces was carried out at Unit 6 of the ICTS Nanbiosis, specializing in the preparation and characterization of nanostructured biomaterials.
For more information, you can consult the full article in ACS Applied Bio Materials.
Reference article:
Activating Thermoplastic Polyurethane Surfaces with Poly(ethylene glycol)-Based Recombinant Human α-Defensin 5 Monolayers for Antibiofilm Activity
Xavier Rodríguez Rodríguez, Adrià López-Cano, Karla Mayolo-Deloisa, Oscar Q. Pich, Paula Bierge, Nora Ventosa, Cristina García-de-la-Maria, José M. Miró, Oriol Gasch, Jaume Veciana, Judith Guasch, Anna Arís, Elena Garcia-Fruitós, Imma Ratera, the FUNCATH investigators
ACS Applied Bio Materials, 20 Feb 2025
DOI: 10.1021/acsabm.4c00732
What is NANBIOSIS?
The goal of NANBIOSIS is to provide comprehensive and integrated advanced solutions for companies and research institutions in biomedical applications. All of this is done through a single-entry point, involving the design and production of biomaterials, nanomaterials, and their nanoconjugates. This includes their characterization from physical-chemical, functional, toxicological, and biological perspectives (preclinical validation).
Leading scientists
The main value of NANBIOSIS is our highly qualified and experienced academic scientists, working in public institutions, renowned universities and other research institutes.
Custom solutions
Designed for either scientific collaboration or the private industry, we adapt our services to your needs, filling the gaps and paving the way towards the next breakthrough.

Cutting-Edge facilities
Publicly funded, with the most advanced equipment, offering a wide variety of services from synthesis of nanoparticles and medical devices, including up to preclinical trials.
Standards of quality
Our services have standards of quality required in the pharmaceutical, biotech and medtech sectors, from Good Practices to ISO certifications.
In order to access our Cutting-Edge Biomedical Solutions with priority access, enter our Competitive Call here.
NANBIOSIS has worked with pharmaceutical companies of all sizes in the areas of drug delivery, biomaterials and regenerative medicine. Here are a few of them:









