26 Dec
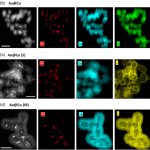
NANBIOSIS Unit 9 advances glioblastoma treatment with tailored copper nanoparticles,[...]
23 Dec

Ibima-plataforma BIONAND has inaugurated an innovative radioactive facility that w[...]

Integrated solutions to advanced challenges faced by biomedical researchers in nanomedicine, tissue engineering and regenerative medicine, diagnostic and medical device, that includes design and production of biomaterials and nanomaterials and their nanoconjugates, and the characterization of these bio-/nanomaterials, tissues and medicals devices from a physic-chemical, functional, toxicological and biological point of view up to preclinical validation.
Complementing the list on the left of “Cutting-Edge Biomedical Solutions” we offer a “Quallity Control and Regulatory Affair Advisory” Service

Customized design and production services of biological molecules for Tissue Engineering, Intelligent Devices, Implants, and specially Therapeutic Nanoconjugates and Biosensors by providing for applications such as:
− Therapeutic agents
− Targeting
− Surfaces functionalization: Tissue engineering/scaffolds & biosensors
Recombinant proteins
Antibodies and Haptens
Custom peptides and post modification
Oligonucleotides
Anti-peptide Antibodies
Protein-only nanomaterial platform for biomedical purposes

Supply nanomaterials for applications in Nanomedicine: therapy, drug delivery, contrast agents (MRI, fluorescence) & theranostic
Nanoparticles
Pure active pharmaceutical ingredients with controlled nansotructure
Supply nanoconjugates for applications in Nanomedicine: therapy, contrast agents (MRI) & theranostic)
Bioconjugation
Nanoconjugation
Micro and nanoencapsulation

Preclinical characterization of nanomedicines including physicochemical properties, in vitro and in vivo biological properties (immunology, toxicology an efficacy with appropriated animal models, at either regulatory (cGLP) or non-regulatory conditions
Complete cascade characterization
Physicochemical characterization
In vitro characterization
In vivo characterization

Development of prototypes and biosensing devices for diagnostic, production of bioreceptor against identified biomarkers an detection of biomarkers for diagnostic, follow-up and prognostic of diseases by NMR spectroscopies in biofluids and tissues.
Development and validation of biosensor
Anti-peptide Antibodies
Devices
Quatintifaction of Biomarker by NMR
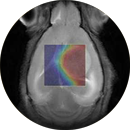
In vivo validation of contrast agents and development of nanoconjugates as contrast agents for MRI and fluorescence.
Nanoparticles
Contrast Agents for MRI & OTC
Validation of NPs for glioma
Validation of Contrast Agents for MRI
Nanocojugates for fluorescence
Nanocojugates for MRI

Design and production of scaffolds for tissue engineering
Scaffolds
Functionalized scaffolds

Preclinical validation of biomaterials including surface and mechanical characterization and in vitro and in vivo biological properties with appropriated animal models, at either regulatory (cGLP) or non-regulatory conditions
Preclinical validation
Surface and mechanical characterization
Adhesion of bateria: biofilms
Biocampatibility

In vivo Validation of medical devices for determined applications in different animal models
Contact us
Platform Unit 1.Biomolecules
|
Platform Unit 2.Biomaterials
|
Platform Unit 3.Preclinical
|
||
|
||||
Units
Scientists linked to ICTS
Projects since 2008
Services since 2008
Publications since 2008
M€ investment