
Revolutionize disease treatment with our new Cutting-Edge Solution — precise enzyme-loaded nanovesicles, offering targeted therapies for various disorders with optimal efficacy and safety.
Scientific Leader / leaders of the project: Nora Ventosa / Ibane Abasolo
Coordinator of the project: Guillem Vargas / Vanessa Díaz-Riascos
Nanovesicles are ideal vehicles for many biomolecules, including enzymes. and might be useful in the treatment of different diseases, such as lysosomal storage disorders (LSD) or severe combined immunodeficiency (SCID). However, the development of such treatments requires a fine understanding of their synthetic processes and their biological impact.
To ensure good efficacy, it is therefore necessary to have a well-defined nanovesicle. For this reason, it is crucial to physicochemically characterize the enzyme-loaded nanovesicles, as these results will have an impact on the efficacy of this treatment. Once the physicochemical parameters have been studied, the functionality of these systems needs to be investigated using appropriate in vivo models.
In this cutting-edge solution, we offer the possibility of producing enzyme-loaded nanovesicles and their full evaluation. The enzyme-loaded nanovesicles will be prepared using the DELOS-SUSP method, which allows the preparation of cholesterol-rich nanovesicles encapsulating some biological and non-biological molecules. The first analysis to perform is the physicochemical characterization, which includes the following characteristics: hydrodynamic diameter, polydispersity index, apparent zeta-potential, particle concentration, colloidal stability, etc. Afterwards, their functionality (enzymatic activity, in vitro and in vivo efficacy) and safety profile (toxicology and immunogenicity) will be tested using relevant animal models.
These properties are crucial to understand the behavior and efficacy of the enzyme-loaded nanovesicles.
Services involved:
The projects are carried out by U6 (Biomaterial Processing and Nanostructuring Unit) and U20 (In Vivo Experimental Platform). There is a strong collaboration between these two platforms. They have participated in several projects and have published several papers together. Both units are involved in the discussion of enzyme-loaded nanovesicles. These nanoparticles are prepared and physicochemically characterised by U6 and the in vivo functionality of the encapsulated enzyme is tested by U20.
1) Use of High-pressure laboratory-scale plant with 50, 100 and 300 m reactors for the processing of biomaterials. Processing of cytotoxic compounds when required. U6-S01.
2) Analysis of particle size, concentration and Z-potential of nanoparticles. U6-S04.
3) In vivo Experimental consultancy. U20-S03.
4) In vivo Efficay Assays of drugs, nanomedicines, biomaterials and others. U20-S04.
5) In vivo Toxicology. U20-S05.
6) In vivo ADME and biodistribution assays. U20-S06.
7) Immunotoxicity assays. U20-S08.
8) Histological processing, section and staining. U20-S11.
Related publications:
Related projects:
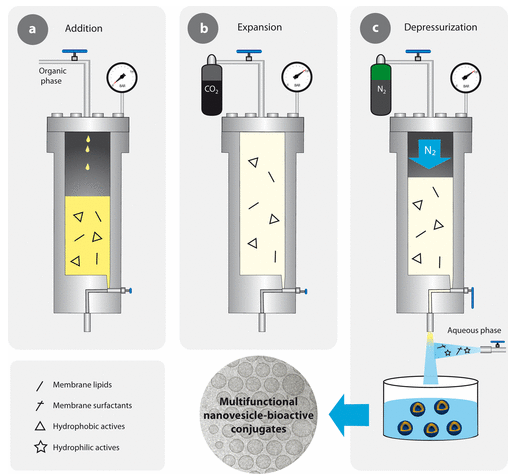 |
Our Biomaterial Processing and Nanostructuring Unit (U6) is located at the Instituto de Ciencia de Materiales de Barcelona (ICMAB-CSIC), in Barcelona, and under the coordination of Professor Jaume Veciana and Prof. Nora Ventosa, current directors of NANOMOL Group, which is a research group with wide expertise and recognized excellence in the synthesis, processing and study of molecular and polymeric materials with chemical, electronic, magnetic and biomedical properties. It gathers several laboratories, perfectly equipped, to perform the mission of this facility: the development, characterization, and large-scale production of molecular biomaterials of therapeutic or biomedical interest, with controlled micro-, nano- and supramolecular structure. One example of Key-Enabling-Technology (KET) available in this unit is a simple one-step methodology, DELOS-SUSP, based on the use of compressed fluids (CF), such as CO2, to prepare particulate materials with precise and reproducible structural characteristics at micro-, nano- and supramolecular levels (size, shape, internal structural gradients, supramolecular organization and crystalline purity). This example shows one of the singularities of this unit is that counts with CF–based plants at different scales, from mL to L, which allow process development by QbD and process scale-up.
The NANBIOSIS Unit 6 is works under the ISO9001 certification for standard quality control system |
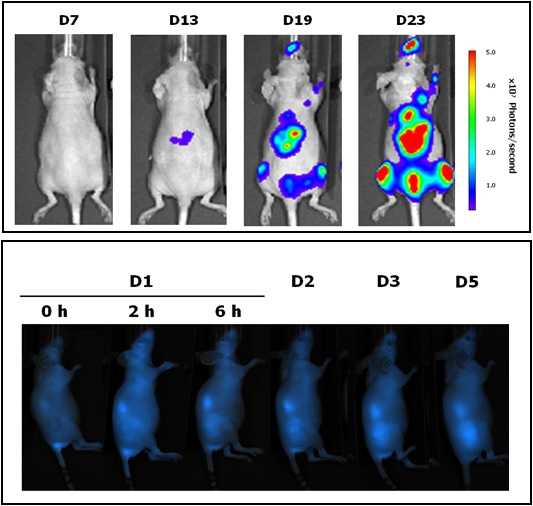 |
Located at the Valld’Hebron Research Institute (VHIR) in Barcelona, our In vivo Experimental Platform (U20) has three different sections, a Molecular Imaging section for in vivo, ex vivo and in vitro imaging studies (fluorescence, bioluminescence and X-rays), a preclinical animal model section and a preclinical histology section. All three sections are included within the Functional Validation & Preclinical Research (FVPR) of CIBBIM-Nanomedicine. This unit is lead by Dr. Ibane Abasolo (Head of FVPR) and supervised by the directorof CIBBIM-Nanomedicine, Dr. Simó Schwartz Jr. Its mission is to offer products and services for the preclinical proof-of-concept validation of therapeutic compounds and biomarkers with potential clinical applications. One of the distinguishing features of this unit is that basic preclinical studies including toxicology, histopathology and efficacy treatments are complemented with non-invasive optical imaging technologies. In vivo bioluminescence and fluorescence imaging allows monitoring of living mice longitudinally, offering real time insight into treatment efficacy, whole-body biodistribution and target mechanisms. Although in Spain there are other institutions with similar equipment, this is the only case in which imaging services can be combined with specifically devoted animal models. |