Characterization and development of pulmonar formulations
3D bio-impression of scaffolding for regenerative medicine
Encapsulation of therapeutic actives of interest in formulations for pulmonary administration with possibility of lyophilisation to obtain a powder. The service has two cutting-edge equipments for the characterization of pulmonary formulations:
SprayTec laser diffraction system: allows the measurement of spray particle and spray droplet size distributions in real-time for more efficient product development of sprays and aerosols, with robust and reproducible droplet size data.
Next Generation Impactor: has been designed specifically for the pharmaceutical industry for testing metered-dose inhalers, drypowder inhalers, nebulizers and nasal sprays. It consists of a high performance cascade impactor for classifying aerosol particles into micrometer size fractions, providing relevant information about their distribution in the respiratory tract.
Customer benefits
The formulations can be characterized, following SOPs, in terms of particle size, polydisperstity index and zeta potential. Importantly, this service can also offer real-time droplet size distribution and aerosol particles classification analysis by means of SprayTec and Next Generation Impactor (NGI) technology.
Target customer
References
Moreno-Sastre M, Pastor M, Esquisabel A, Sans E, Viñas M, Fleischer A, Palomino E, Bachiller D, Pedraz JL. Pulmonary delivery of tobramycin-loaded nanostructured lipid carriers for Pseudomonas aeruginosa infections associated with cystic fibrosis. Int J Pharm. 2016 Feb 10;498(1-2):263-73. doi: 10.1016/j.ijpharm.2015.12.028.
Additional information
The principle of 3D bioprinting consists of selecting the most suitable biomaterials and cell types to prepare a Bioink that should be able to promote cell growth and differentiation and present appropriate mechanical properties of the target tissue.
This service possess a wide variety of 3D bioprinting techniques avaliable, such as extrusion, droplet, electrospining, electrowritting and stereolithography.
Customer benefits
One of the main characteristics of this additive manufacturing technique is its ability to bioprint the desired layers, with specific cell orientation , and desired morphology of the bioprinted 3D scaffold in order to ressemble, as much as possible, the tissue of interest. To achieve this goal, rheology, texturometry, printability and biological assays are carried out.
On the one hand, this technology can be employed to develop 3D scaffolds specific for the regeneration of particular tissues. On the other hand, this strategy offers a 3D environment that mimics the tissue/ organ of interest in order to test potential therapeutic tools, which goes in accordance with the implementation of the 3R principle (replace, reduce and refine).
Target customer
References
Additional information
3D Bioprinters: BIO X 3D Bioprinter –CELLINK (left); R-GEN 100 –REGENHU (right).
Bioprinted 3D scaffold
1) NGI Cascade Impactor: It’s an analytical tool for the development of inhalation products. It’s use for the testing of all inhalation formulations and devices: MDIs, DPIs, nebulizers and aerosol and nasal sprays. It’s the new “workhorse” of the pharmaceutical industry.
2) Alberta Idealised Throat (AIT): before entering the lungs, aerosols must traverse the mouth-throat. So it’s important to be able to mimic aerosol deposition and flow in the human mouth-throat when studying inhaled aerosols. The Alberta geometry has been shown to do a remarkable job of mimicking the aerosol and flow motion in the human mouth-throat and allow researchers examining and testing aerosols inhaled orally.
3) Breath Simulator: are increasingly used in testing orally inhaled and nasal drug products (OINDPs) to replace existing constant flow conditions with breathing profiles more representative of conditions in vivo. It can be used for improved IVIVC applications, with real breath profiles generated in clinic.


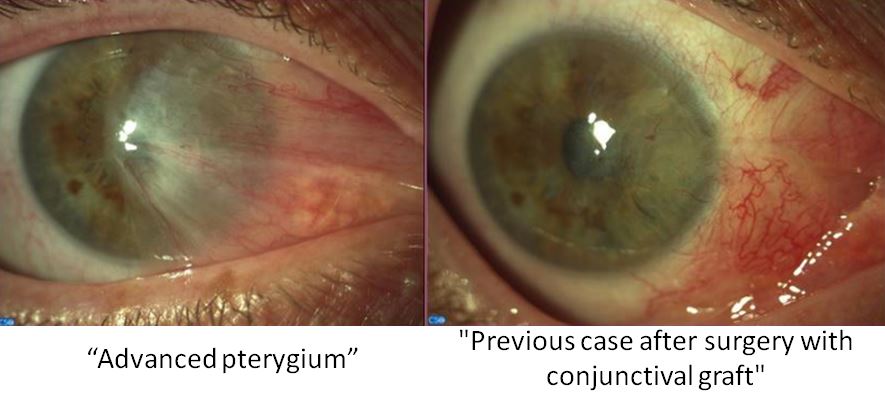
Dr. José Luis Pedraz, head of Nanobiocel group and Scientific Director of Unit 10 of NANBIOSIS, participates in the public-private collaboration BIOTAPE project that will develop a new bioadhesive for its application in pterygium surgery to improve the currently used surgical techniques in the treatment of this eye disease that affects the conjunctiva and cornea.
The main cause of pterygium is the lack of lubrication of the eye by the tear film. This lack of lubrication may be due to various external factors such as excessive exposure to sunlight, harsh environmental conditions or even allergens, which causes dryness and subsequent irritation in that area. Pterygium grows in the form of fleshy mass on the cornea, being in these cases the application of surgery to extract it. So far the technique with better results is to place a graft of the conjunctiva of the eye itself in the place of removal of the pterygium, fixed by sutures or tissue adhesives.
In the BIOTAPE project, 3D printing technique will be used to manufacture the new bioadhesive that will help reduce the current complications among which we can highlight the high rates of recurrence of the disease, surgical and post-surgical complications and the cosmetic result, not always satisfactory.
The new project, to be developed over the next three years, is funded with a budget of 513,000 euros by the Challenges-Collaboration program of the Ministry of Economy, Industry and Competitiveness and the participation of three Spanish companies (AJL OPHTALMIC, BRECA Health Care and Bilboftal-ICQO), the University of Miguel Hernández in Elche, CIBER-BBN and unit 10 of NANBIOSIS-ICTS.
“Promover el desarrollo tecnológico, la innovación y la investigación de calidad”
Climate chambers are needed because our laboratory is able to carry out stability studies in climatic chambers under ICH conditions and with access to Good Laboratory Practice (GLP).

