U24-S01 Evaluation of therapies for cardiovascular disease
Evaluation of therapies for cardiovascular disease
With cardiovascular disease consistently representing a major cause of death worldwide, a platform to perform experimental studies testing the efficacy of candidate therapies for CVD is necessary.
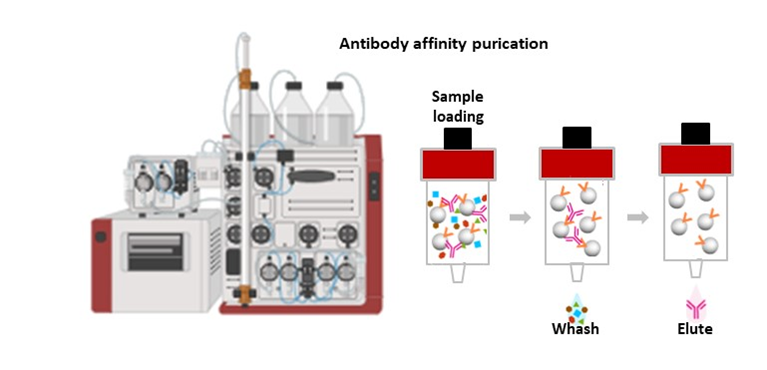
The methodology implemented in NANBIOSIS Unit 24 for this purpose has been tested and validated in several papers (Refs below). In brief, CVD is induced in a relevant large animal model (i.e. myocardial infarction and swine) using image guided surgical techniques (i.e. percutaneous balloon occlusion of a coronary artery for a pre-determined amount of time). Once the model is established, the therapy under study is applied and the experimental subjects followed up for a fixed length of time. Clinical grade imaging (i.e. Cardiac Magnetic Resonance including delayed enhancement) and laboratory techniques are used to follow up and document the evolution of the induced CVD. Generally, image acquisition is performed at baseline and serially during the predetermined duration of the study in order to study the effect of the therapy on measurable endpoints (i.e. left ventricular ejection fraction) to document improvement.
Customer benefits
The studies are tailored to the needs of each specific candidate intervention, can be implemented with different follow-up times and can be performed under regulatory requirements, since the performing institution is Certified for Good Laboratory Practices.
Thus the Service can be provided as proof-of-concept studies, full safety and efficacy or under GLPs to meet regulatory agencies’ guidelines and assure clinical translation, so that the customers can take their therapy to a clinical trial faster and more efficiently, thanks to the full range of capabilities offered in this service.
Target customer
The offered service can be of interest to scientists from academia willing to test a possible CVD therapy, including biologicals, small companies that have a candidate molecule which is promising enough to warrant large animal trials or big pharma willing to undergo GLP studies to commercialize their product.
References
- Aimo A et al. Colchicine added to standard therapy further reduces fibrosis in pigs with myocardial infarction. J Cardiovasc Med (Hagerstown). 2023 Nov 1;24(11):840-846. doi: 10.2459/JCM.0000000000001554. Epub 2023 Sep 29. PMID: 37773884.
- Österberg K et al. Personalized tissue-engineered veins – long term safety, functionality and cellular transcriptome analysis in large animals. Biomater Sci. 2023 May 30;11(11):3860-3877. doi: 10.1039/d2bm02011d. PMID: 37078624.
- Pulido M et al. Transcriptome Profile Reveals Differences between Remote and Ischemic Myocardium after Acute Myocardial Infarction in a Swine Model. Biology (Basel). 2023 Feb 21;12(3):340. doi: 10.3390/biology12030340. PMID: 36979032; PMCID: PMC10045039.
- Blanco-Blázquez V et al Intracoronary Administration of Microencapsulated HGF in a Reperfused Myocardial Infarction Swine Model. J Cardiovasc Dev Dis. 2023 Feb 17;10(2):86. doi: 10.3390/jcdd10020086. PMID: 36826582; PMCID: PMC9960949.
- Arenal Á et al. Effects of Cardiac Stem Cell on Postinfarction Arrhythmogenic Substrate. Int J Mol Sci. 2022 Dec 19;23(24):16211. doi: 10.3390/ijms232416211. PMID: 36555857; PMCID: PMC9781106.
- Báez-Díaz C et al. Intrapericardial Delivery of APA-Microcapsules as Promising Stem Cell Therapy Carriers in an Experimental Acute Myocardial Infarction Model. Pharmaceutics. 2021 Nov 1;13(11):1824. doi: 10.3390/pharmaceutics13111824. PMID: 34834235; PMCID: PMC8626005.
- Crisóstomo V et al. The epicardial delivery of cardiosphere derived cells or their extracellular vesicles is safe but of limited value in experimental infarction. Sci Rep. 2021 Nov 12;11(1):22155. doi: 10.1038/s41598-021-01728-y. PMID: 34772964; PMCID: PMC8590017.
- Prat-Vidal C et al. Intracoronary Delivery of Porcine Cardiac Progenitor Cells Overexpressing IGF-1 and HGF in a Pig Model of Sub-Acute Myocardial Infarction. Cells. 2021 Sep 28;10(10):2571. doi: 10.3390/cells10102571. PMID: 34685551; PMCID: PMC8534140.
- Ziani K et al. Characterization of encapsulated porcine cardiosphere-derived cells embedded in 3D alginate matrices. Int J Pharm. 2021 Apr 15;599:120454. doi: 10.1016/j.ijpharm.2021.120454. Epub 2021 Mar 5. PMID: 33676988.
- Rossello X et al. CIBER-CLAP (CIBERCV Cardioprotection Large Animal Platform): A multicenter preclinical network for testing reproducibility in cardiovascular interventions. Sci Rep. 2019 Dec 30;9(1):20290. doi: 10.1038/s41598-019-56613-6. PMID: 31889088; PMCID: PMC6937304.
- Crisostomo V et al. Dose-dependent improvement of cardiac function in a swine model of acute myocardial infarction after intracoronary administration of allogeneic heart-derived cells. Stem Cell Res Ther. 2019 May 31;10(1):152. doi: 10.1186/s13287-019-1237-6. PMID: 31151405; PMCID: PMC6544975.