NANOMEDICINE APPLICATIONS IN DRUG DELIVERY AND TARGETING: NANBIOSIS – NANOMED Industrial Forum
Yesterday took place in Barcelona, at Barcelona School of Management, Universitat Pompeu Fabra, a meeting of resarch groups and units of NANBIOSIS and CIBER-BBN and companies in the third B2B Forum organized by NANBIOSIS, in this case together with NANOMED SPAIN.
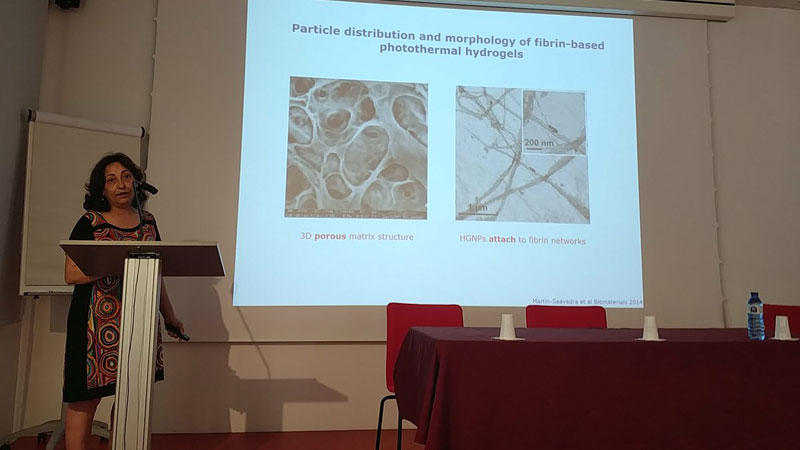
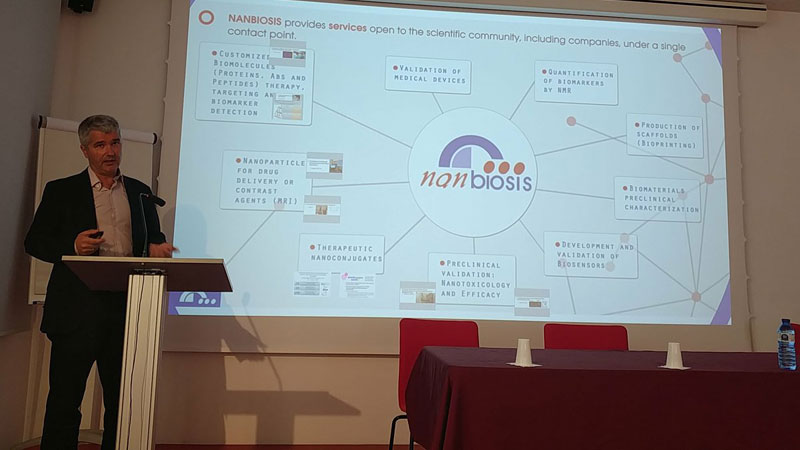
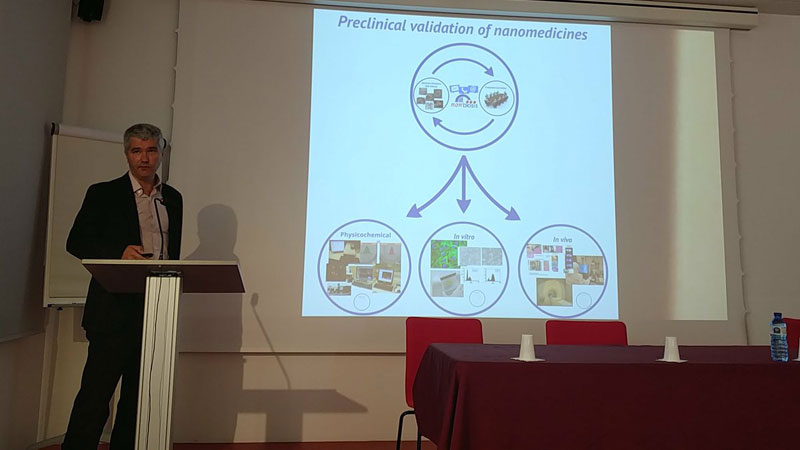
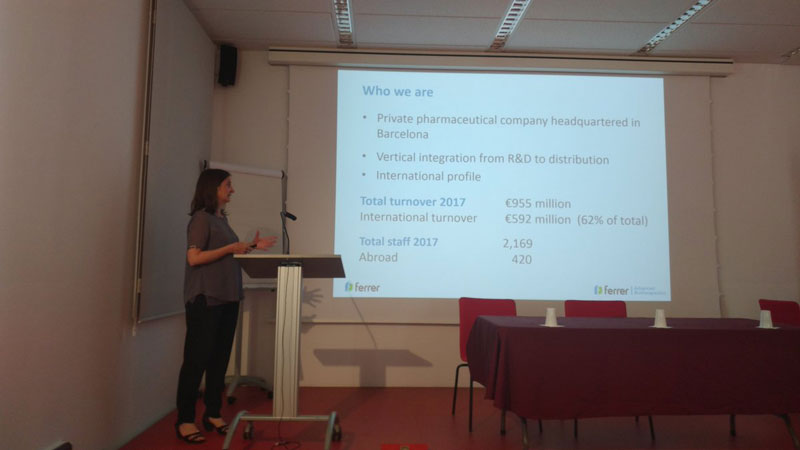
Thirteen companies and twelve groups from CIBER-BBN and CCMIJU (ten of them coordinating NANBIOSIS units) got together to explain, through short presentations of ten minutes, those lines of their work aimed at finding synergies and potential collaborations in the area of Nanomedicine apllications in drug delivery and targeting. There was also a talk by a representative of CDTI (Spanish National Center for Industrial and Technological Development) to explain the financing opportunities for the companies as well as a presentation by the NANBIOSIS Coordinator, Jesús Izco, to show the new Cutting Edge Biomedical Solutions offered by the ICTS-NANBIOSIS.
After lunch, the groups and companies had the opportunity to discuss in more detail, during bilateral interviews coordinated by NANBIOSIS a, those aspects that had attracted their attention, as well as, in some cases, to draw potential collaborations. The event was successfully developed with 45 attendees and more than 50 individual B2B mettings.