Smart-4-Fabry final workshop
Next Wedneday, February 3, 2021 will take place the on-line event Smart-4-Fabry Final Workshop.
Smart-4-Fabry is a european project, coordinated by CIBER-BBN wich has been developed during four years. This project is a sign of cooperation at European level to boost nanomedicine development and translation to clinical stages.
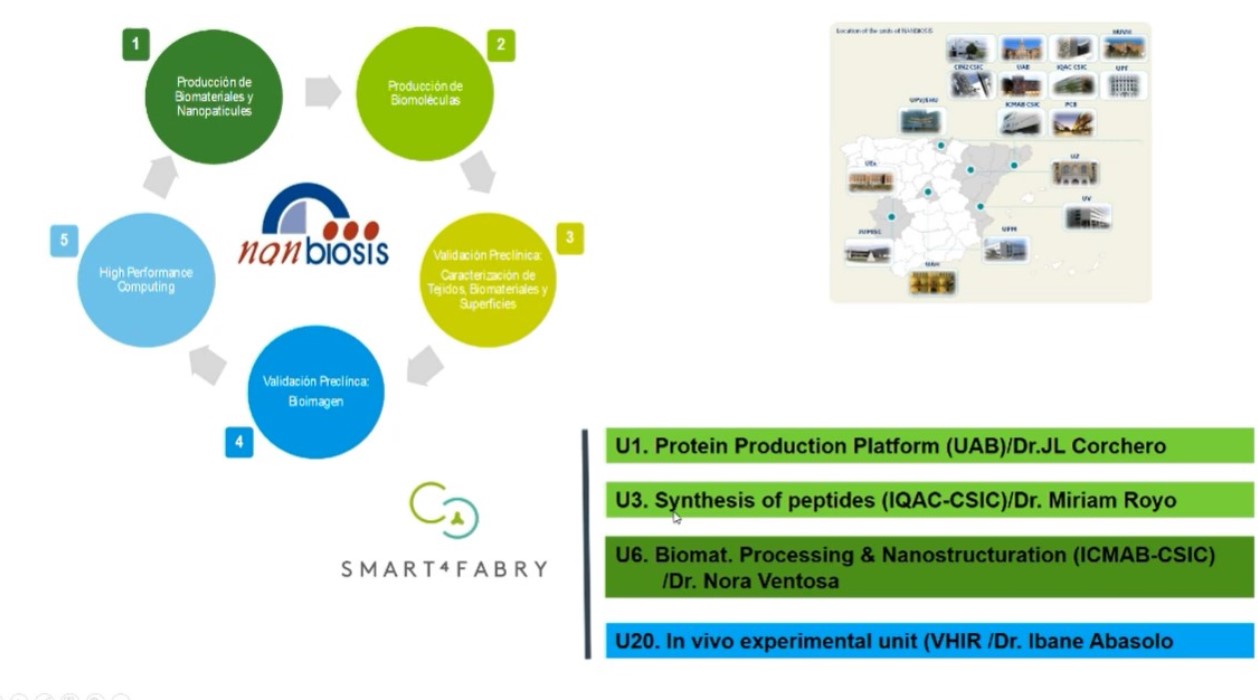
This project is also a clear example of the relevance of access to advanced research infrastructures as NANBIOSIS -ICTS. Four NANBIOSIS units have collaborated and contributed to Smart-4-Fabry development:
- U1 Protein Production Platform (PPP) at IBB-UAB
- U3 Synthesis of Peptides Unit at IQAC-CSIC
- U6 Biomaterial Processing and Nanostructuring Unit at ICMAB-CSIC
- U20 In Vivo Experimental Platform at Vall d’Ebron Hospital
“The Fabry disease (FD) is a lysosomal storage disorder (LSD) that currently lacks an effective treatment” as Prof. Nora Ventosa, IP of the project, explained for NANBIOSIS blog – The aim of Smart-4-Fabry is to obtain a new nanoformulation of GLA, that will improve the efficacy and toleration compared to the actual treatment with non-formulated GLA.
In the final workshop experts will talk about how, why and for what the solution proposed by Smart4Fabry was conceived.
Registrations and program at https://smart4fabry.cientifis.com/