How a tumor metabolite changes Glioma behavior and improves treatment response
A new study shows how a tumor metabolite can make aggressive glioma cells more sensitive to treatment, revealing new opportunities for therapy.
Valencia, February 2026 — A recent study published in Biomedicines shows that D-2-hydroxyglutarate (2HG), a molecule produced by certain brain tumors, can significantly change how glioma cells behave. The research demonstrates that when IDH-wildtype glioma cells are exposed to 2HG, they begin to resemble IDH-mutant tumors, becoming less aggressive and more sensitive to standard cancer treatments. The study involved the use of NANBIOSIS research infrastructure (more specifically, Unit 26), underlining its role in advanced biomedical research.
The article, titled “Isocitrate Dehydrogenase-Wildtype Glioma Adapts Toward Mutant Phenotypes and Enhanced Therapy Sensitivity Under D-2-Hydroxyglutarate Exposure” (Biomedicines, 2025), explores how tumor metabolism influences the growth, spread, and treatment response of gliomas.
What are IDH mutations and why do they matter?
Some gliomas carry mutations in a gene called isocitrate dehydrogenase (IDH). These mutations cause tumor cells to produce 2HG, a so-called “oncometabolite” that alters how cells grow and function. Interestingly, patients with IDH-mutant gliomas often respond better to treatment, but the reasons behind this have not been fully understood.
To better understand this effect, researchers compared glioma cells with and without IDH mutations. They studied how the cells behaved under low-oxygen conditions, which are common inside tumors, and how they responded to chemotherapy (temozolomide) and radiotherapy.
What did the researchers find?
The study (full reference at the end of this piece) found clear differences between the two types of tumor cells:
- IDH-wildtype glioma cells grew faster and adapted more easily to low oxygen levels
- IDH-mutant cells divided more slowly and showed signs of growth arrest
When IDH-wildtype cells were exposed to 2HG, however, they began to change. These cells showed slower growth, increased cell death during chemotherapy, and changes in surface markers linked to tumor aggressiveness. In other words, 2HG made aggressive tumor cells behave more like the less aggressive IDH-mutant tumors.
Evidence from animal models
The researchers also tested these effects in mouse models of glioma. Tumors formed from IDH-wildtype cells were larger and spread more into surrounding brain tissue. In contrast, tumors exposed to 2HG were smaller and more compact.
Although some tumor cells became more mobile over time, this movement was strongly reduced when chemotherapy or radiotherapy was applied. This suggests that 2HG exposure may increase the effectiveness of existing treatments.
These findings show that tumor metabolism plays a key role in how gliomas grow and respond to therapy. While IDH mutations and 2HG may initially help tumor cells survive, they also appear to make tumors more vulnerable to treatment and less invasive.
Understanding this balance could help researchers identify new treatment strategies that exploit these weaknesses, potentially improving outcomes for patients with glioblastoma and other gliomas.
NANBIOSIS infrastructure supporting the study
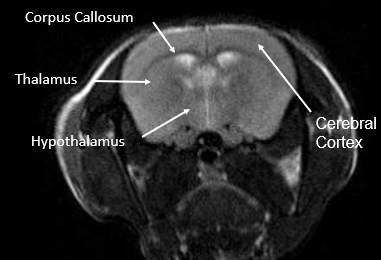
This research involved the use of NANBIOSIS Unit 26 (U26. NMR: Biomedical Applications II). The BMRI-3T (MR Solutions) system, registered at the Unit 26 facility of ICTS NANBIOSIS at the Universitat de València, was used for in vivo imaging studies. The authors also acknowledged Musta Ezzedin Ayoub from the UCIM–Universitat de València imaging team for his contribution to image acquisition.
NANBIOSIS Unit 26 is based at the Faculty of Medicine of the University of Valencia and provides advanced NMR and imaging technologies for biomedical research. The Unit supports studies on metabolism, disease mechanisms, and treatment response in both academic and industrial projects.
Read the full paper here.
What is NANBIOSIS?
The goal of NANBIOSIS is to provide comprehensive and integrated advanced solutions for companies and research institutions in biomedical applications. All of this is done through a single-entry point, involving the design and production of biomaterials, nanomaterials, and their nanoconjugates. This includes their characterization from physical-chemical, functional, toxicological, and biological perspectives (preclinical validation).
If you want to collaborate with us, visit our Order Request page.
In order to access our Cutting-Edge Biomedical Solutions with priority access, enter our Competitive Call here.
NANBIOSIS has worked with pharmaceutical companies of all sizes in the areas of drug delivery, biomaterials and regenerative medicine. Here are a few of them: