A new smart drug that finds and kills metastasis cells could be applied in 23 types of cancer
Researchers of two CIBER-BBN Units of the ICTS NANBIOSIS U18 Nanotoxicology Unit at Hospital Sant Pau. and U1, Protein Production Platform (PPP), at the Institute of Biotechnology and biomedicine of the Autonomous University of Barcelona (IBB–UAB), led by Prof Ramón Mangues, have developed a new drug that selectively removes metastatic stem cells, inducing a powerful metastasis prevention effect.
Besides the participation of the “NANBIOSIS” ICTS Units
U1 Protein Production Platform where Protein production was partially performed and U18 Nantoxicology Unit where Biodistribution studies were performed, all in vivo experiments were performed by the Unit 20 In Vivo Experimental Platform of CIBER in Bioengineering, Biomaterials & Nanomedicine (CIBER-BBN)
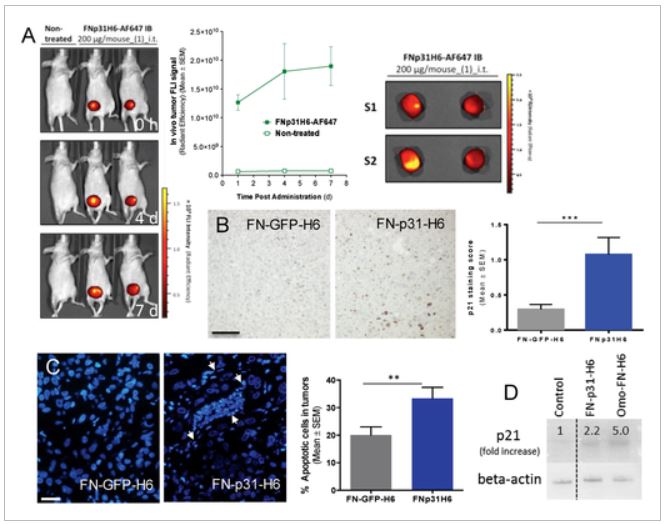
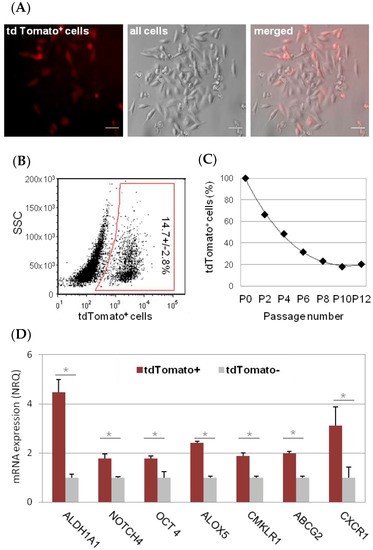
The researchers have ceated inclusion bodies of amyloid and nanostructured fibers that, when administered subcutaneously in mice, release soluble cytotoxic nanoparticles continuously. These nanoparticles are carriers of the exotoxin of Pseudomonas aeruginosa that manages to maintain a stable concentration of this nanomedicine in the blood and tissues. Dr. Mangues explains that “this new pharmaceutical form of subcutaneous administration for sustained release allows high doses of this nanopharmaceutical to be administered, at prolonged intervals (weeks in mice and probably months in humans) without toxicity at the injection site or in normal tissues, while generating a powerful antimetastatic effect. Apart from being controlled-release systems, these nanoparticles incorporate a ligand that interacts with the receptor (CXCR4), present at high levels in the membrane of metastatic stem cells capable of generating metastases (CMM CXCR4 +). Once the new pharmaceutical form is administered subcutaneously in mice with metastatic colorectal cancer, this ligand directs each nanoparticle released by this structure to the tumor tissues, increasing their uptake, to specifically internalize in the CXCR4 + CMMs and induce their selective destruction. “This effect achieves a notable reduction in tumor size in the colon while blocking the development of lymph node, lung, liver and peritoneal metastases, without appreciable uptake or toxicity in non-tumor tissues” continous the researchers.
The researchers estimate that this new therapeutic strategy will have a high clinical impact by reducing the requirement of its hospital administration, which most antitumor drugs have, and blocking metastatic dissemination, responding to an unmet clinical need. On the other hand, this new pharmaceutical form, which combines sustained release with targeting to the CXCR4 receptor, could be used in the treatment of at least 23 types of cancer that also express high levels of this receptor in tumor cells.
The new therapy offers an answer to the urgent medical need to inhibit the development of metastases, which represents the leading cause of death in cancer patients. The selective destruction of tumor and metastatic cells increases the therapeutic index of nanomedicine, obtaining a potent antimetastatic effect without generating associated adverse effects, which differentiates it from most of the currently used antitumor drugs.
Article of reference
María Virtudes Céspedes, Olivia Cano‐Garrido, Patricia Álamo, Rita Sala, Alberto Gallardo, Naroa Serna, Aïda Falgàs, Eric Voltà‐Durán, Isolda Casanova, Alejandro Sánchez‐Chardi, Hèctor López‐Laguna, Laura Sánchez‐García, Julieta M. Sánchez, Ugutz Unzueta, Esther Vázquez, Ramón Mangues, Antonio Villaverde. Engineering Secretory Amyloids for Remote and Highly Selective Destruction of Metastatic Foci Adv.Mater.2019, 1907348