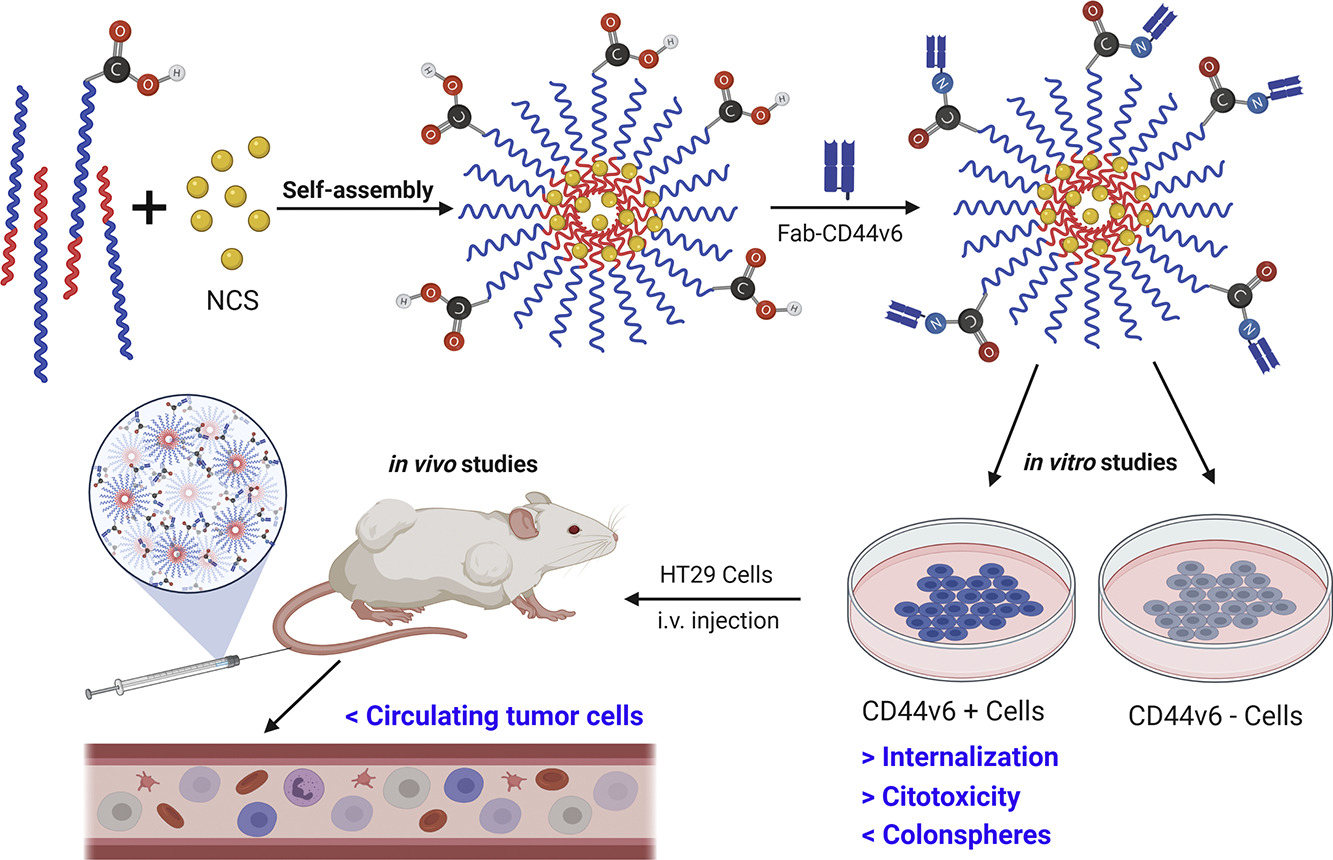
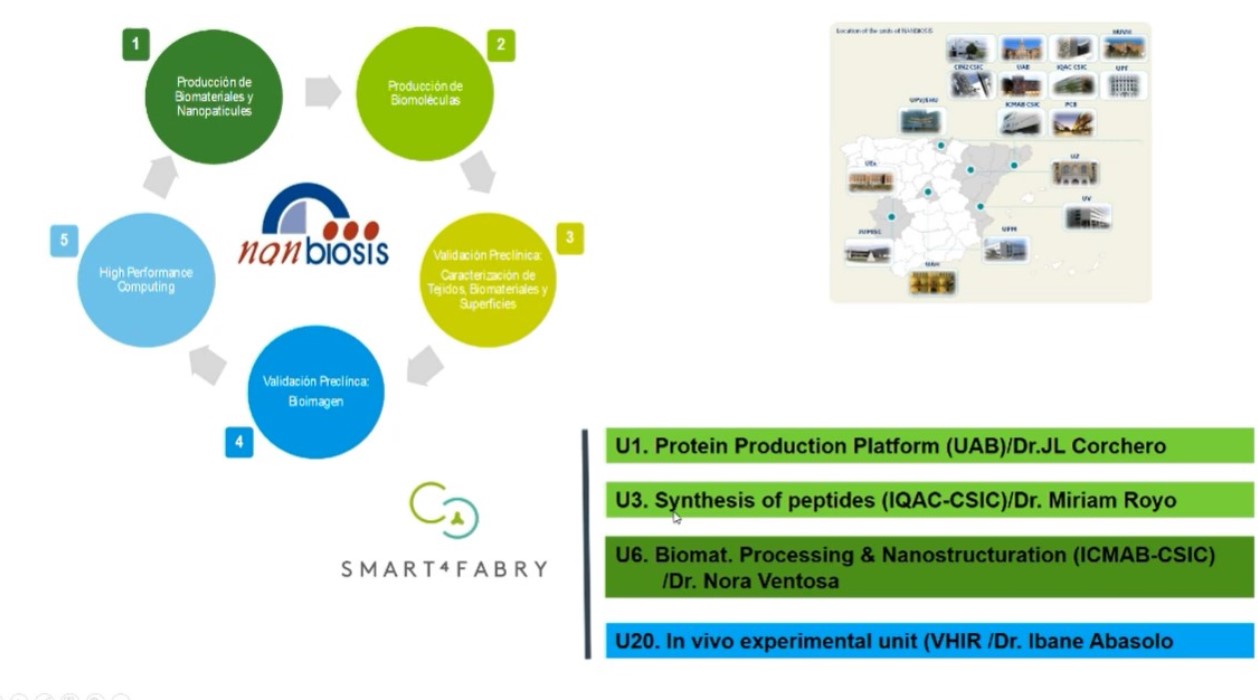
Simo Schwartz: new General Director of the Bank of Blood and Tissues of Catalonia.
Simó Schwartz Jr., Scientific Director of NANBIOSIS Unit 20 In Vivo Experimental Platform has been appointed as the new general director of the Bank of Blood and Tissues of Catalonia, replacing Enric Argelagués, who is leaving his post after 17 years as director. Argelagués was the promoter of the unification of blood banks that began in the eighties and culminated in the current Bank of Blood and Tissues of Catalonia.
Simó Schwartz now assumes the executive management of the organization, which currently has more than 800 workers and its mission is to ensure that all people in Catalonia have the blood and tissues necessary for their treatment at their disposal, promoting proper use. It is a reference center in diagnostic immunology and in the development of advanced therapies, it is present in the main hospitals in Catalonia and is part of the national and international organizations related to donation, transfusion and treatments with biological components.