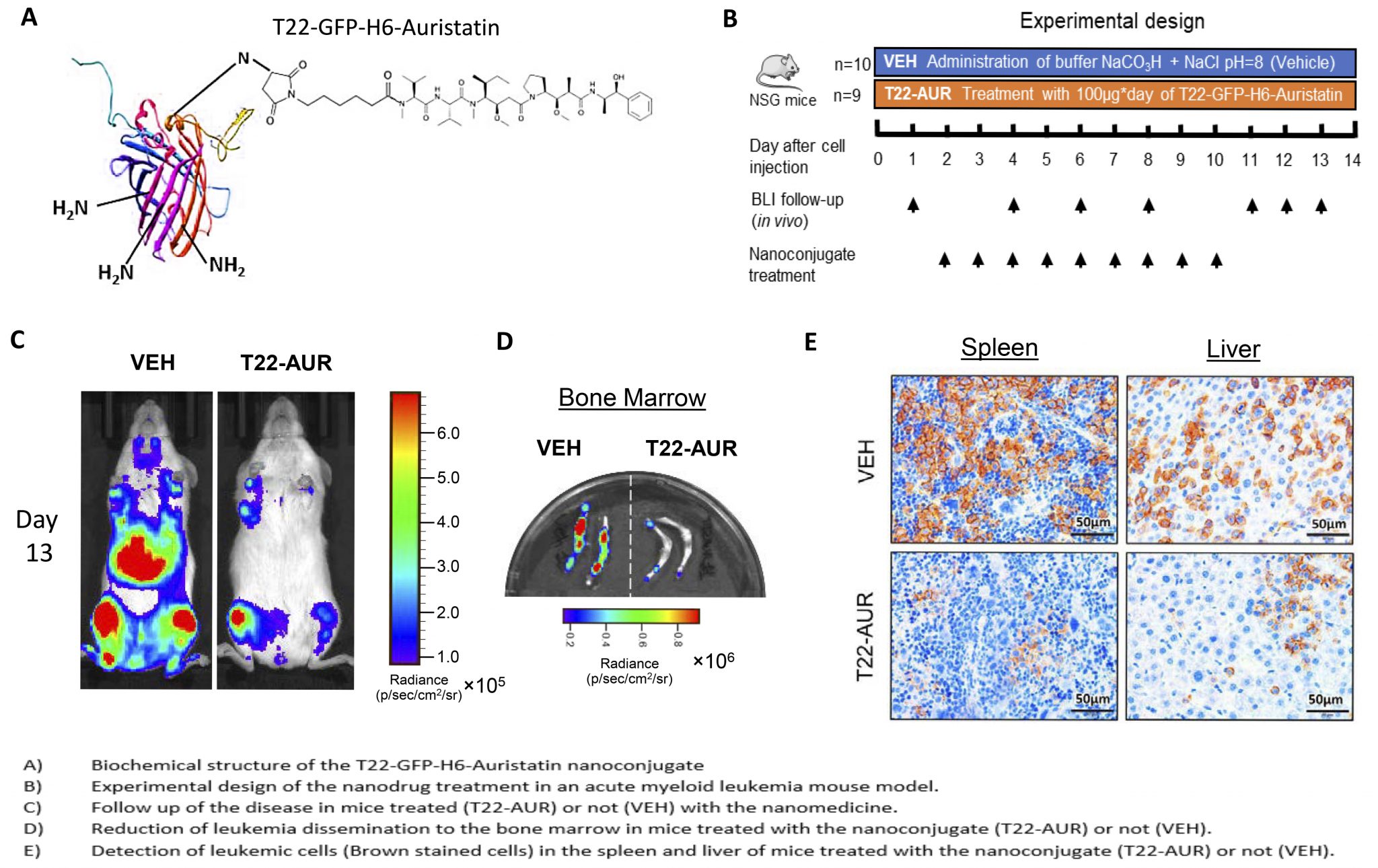
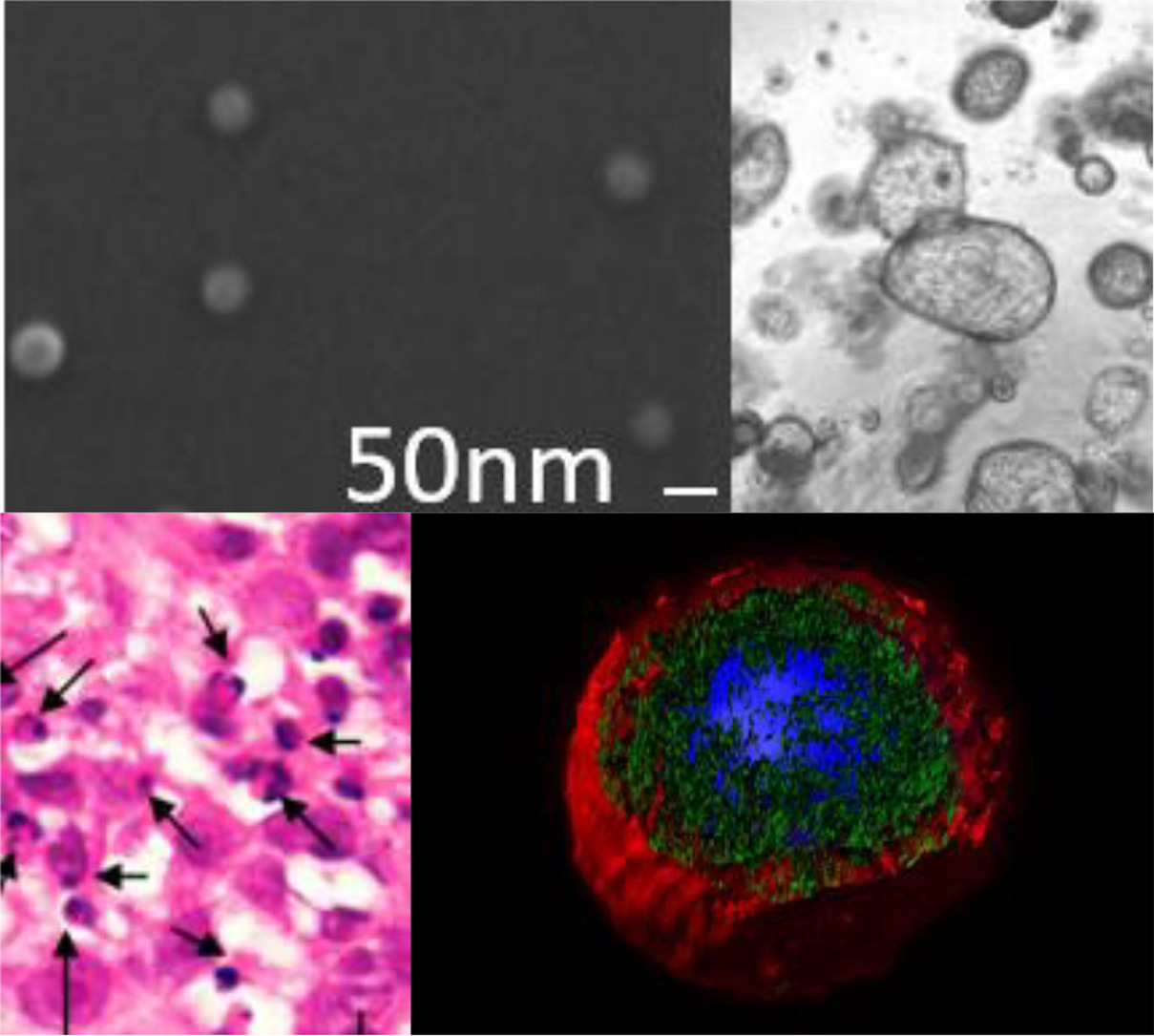
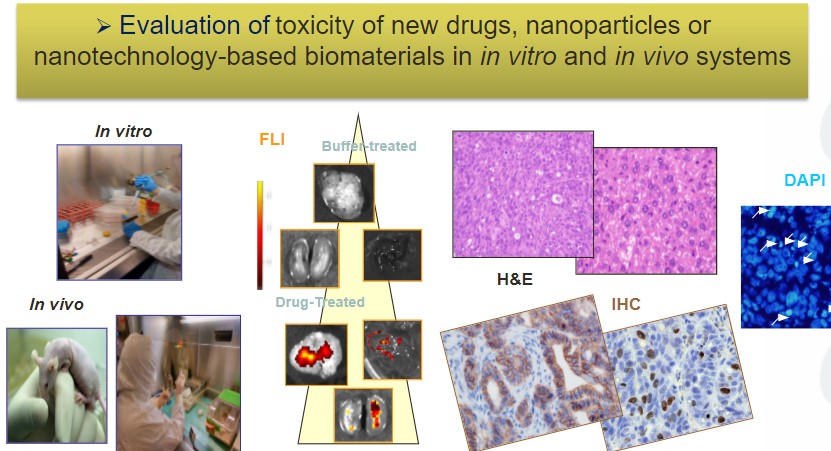
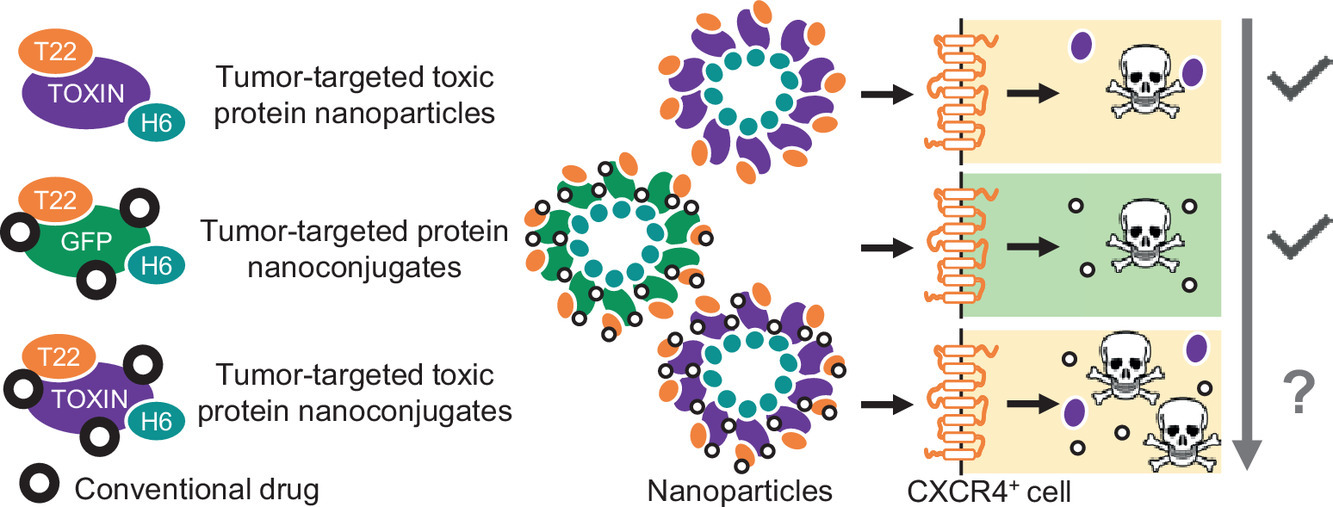
Dr Ramón Mangues, new member of the Royal Academy of Pharmacy of Catalonia
Dr. Ramon Mangues, head of the Oncogenesis and Antitumor Group and Scientific Director of NANBIOSIS U18 Nanotoxicology Unit of CIBER-BBN at the Sant Pau Research Institute, has been recently elected as a new member of the Royal Academy of Pharmacy of Catalonia.
The celebration will take place on November 8 at 7 pm, at the headquarters of the RAFC, (Royal Academy of Pharmacy of Catalonia) which was the headquarters of the old Hospital de la Santa Cruz since the 15th century, located at Calle del Hospital, 56, in Barcelona.
During the event, Dr. Mangues will read his admission speech “Selective delivery of drugs to metastatic stem cells“, which can be followed by zoom and live on Youtube using the following links: