New gelatine devices that mimic the body’s activity in bone regeneration
The NanoBioCel Group, Coordinator of Unit 10 of NANBIOSIS has led the development of new scaffolds (such as burns, trauma or tumour extractions), to regenerate critical bone defects that, in addition to physical support, offers the opportunity to release growth factors temporarily replacing the bone matrix and aiding the regeneration of bone tissue.
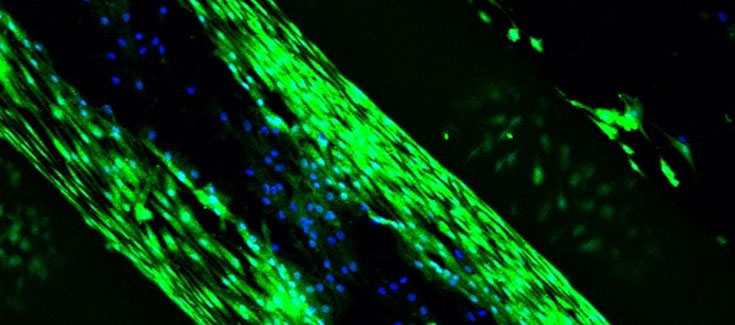
In order to make the material biodegradable, and reduce the risk of rejection, “we use a collagen derivative, a gelatine that is produced by processing collagen, since it has been found to be less cytotoxic than collagen itself, but maintains the properties we were looking for”, explains Pello Sánchez, member of the NanoBioCel group. In addition, for the polymerization of gelatine proteins and scaffold cohesion, they used a molecule that is extracted from the fruit of gardenia, genipina, “because it has a lower toxicity to the cells.”
All the tests and processes carried out to know the properties, biocompatibility and possible cytotoxicity of the scaffolds have been satisfactory. Preclinical studies have been performed on animals with promising results, which are in the process of being published. The group is trying now to improve what has been achieved to date, such as introducing other elements such as calcium, or other growth factors, that improve regeneration.
The project is part of a new line of research promoted by Drs. Gorka Orive and José Luis Pedraz, whose research group NanoBioCel of the Laboratory of Pharmacy and Pharmaceutical Technology of the UPV / EHU and CIBER-BBN coordinates Unit 10 of NANBIOSIS, used in the research. They also have counted with the collaboration of UCA (Unit of Arthroscopic Surgery), and the work of the company AGRENVEC, which was the supplier of the growth factors.
Bibliographic referenc
Sánchez, J.L. Pedraz, G. Orive .. Biologically active and biomimetic dual gelatin scaffolds for tissue engineering. International Journal of Biological Macromolecules, 98: 486-494 (2017). DOI: 10.1016 / j.ijbiomac.2016.12.092.