How to delay vision loss in hereditary retinal dystrophies? Looking for the most effective and economical pharmacological nanotherapy
Researchers from the CIBER-BBN and NANBIOSIS are participating in a new project that aims to achieve the most effective, specific and economical pharmacological nanotherapy that allows delaying the death of retinal cells and subsequent loss of vision in hereditary retinal dystrophy. retinitis pigmentosa, regardless of the genetic defect causing the disease.
In this project, coordinated by the researcher Regina Rodrigo, the ONCE and the Prince Felipe Research Center (CIPF) of Valencia collaborate, together with researchers from the CIBER of Rare Diseases (CIBERER) and the Manises Hospital in Valencia. On behalf of the CIBER-BBN, the scientific director, Ramón Martínez Máñez, together with Elena Aznar from the IDM-UPV group of the Polytechnic University of València, José Luís Pedraz, Gustavo Puras and Idoia Gallego, from the group of the University of the Basque Country and Nanbiosis.
The NANBIOSIS participation in the project will be through the U10 Drug Formulation unit (from @CIBERBBN and @upvehu), led by NanoBioCell Group and Prof. José Luis Pedraz and U26 NMR: Biomedical Applications II , led by IDM-UPV-UV Group, led by Prof. Ramón Martínez Máñez. Elena Aznar, researcher of CIBER-BBN at IDM-UPV-UV explained “We use the unit to characterize the nanoparticles. Through a solid phase NMR confirms that the molecular gate has been correctly attached to the surface of the nanoparticles“.
Retinitis pigmentosa is a group of inherited retinal dystrophies characterized by progressive and irreversible loss of vision. Although it is considered a rare disease, it is the leading genetic cause of blindness in developed countries. So far there is no effective treatment, although there are various therapeutic approaches such as gene therapy, cell therapy, pharmacological therapy, optogenetics or electronic implants.
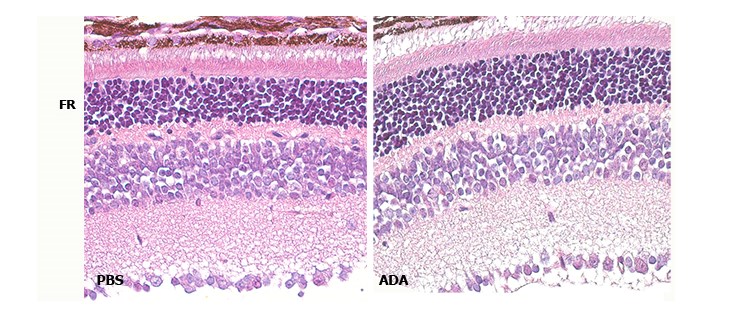
During the progression of the disease, an important inflammatory component has been observed that may contribute to its pathogenesis. In this sense, different anti-inflammatory strategies have been evaluated. The research group has successfully tested one of these strategies in preclinical models of retinitis pigmentosa. However, the implementation of this therapeutic strategy with nanocarriers as controlled release delivery systems would improve the mode of action of the administered drug, avoiding its degradation, increasing its half-life, stability or its availability in the retina. In this project, two types of nanocarriers will be used and their effect on the degenerative process in a murine model of retinitis pigmentosa will be evaluated.
- PICTURE: Hematoxylin and eosin image showing that intravitreal blockade of the cytokine TNFα with Adalimumab-type monoclonal antibodies (ADA) reduces retinal degeneration, preventing the death of photoreceptors (RF) in the murine model of retinitis pigmentosa, rd10 mouse.