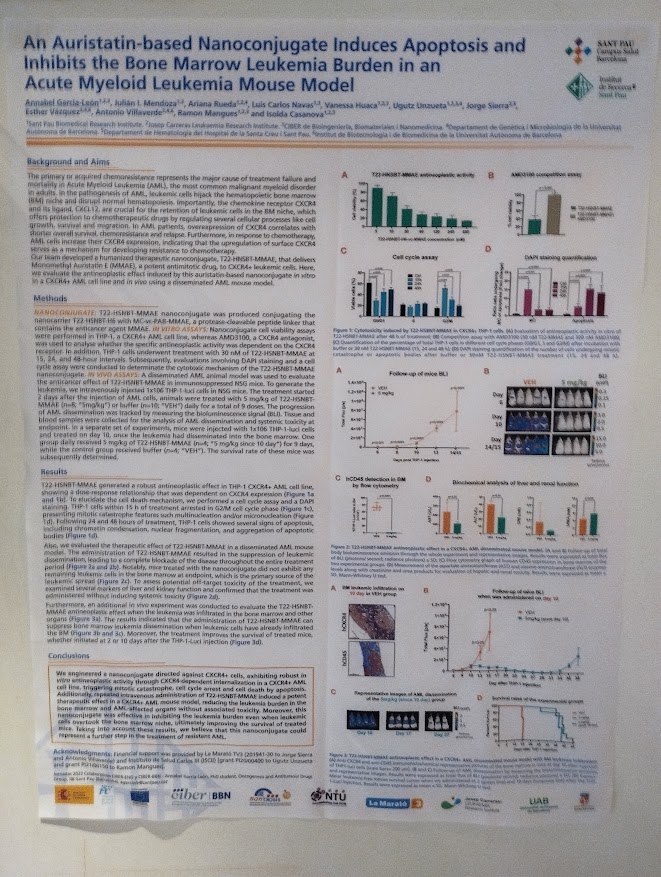
Innovative eye drops offer new hope for Retinitis Pigmentosa patients
NANBIOSIS Unit 10 helps develop eye drops with adalimumab for retinitis pigmentosa, showing promising results in preclinical models.
Vitoria, april 2025. A multidisciplinary team of researchers from the Centro de Investigación Biomédica en Red (CIBER), the Centro de Investigación Príncipe Felipe (CIPF), and the University of the Basque Country (UPV/EHU) has developed an innovative eye drop formulation as a potential treatment for retinitis pigmentosa, a hereditary retinal disease that currently lacks effective therapies for most patients. The findings have been published in the prestigious journal Biomedicine & Pharmacotherapy, as reported by CIBER.
The study was led by Regina Rodrigo (CIPF) with significant contributions from Prof. José Luis Pedraz and Dr. Idoia Gallego Garrido, leaders of Unit 10 of NANBIOSIS – Drug Formulation Unit, based at UPV/EHU. The NANBIOSIS platform provided key resources and technological support for the development and physicochemical characterization of the therapeutic formulation.
Therapeutic antibodies
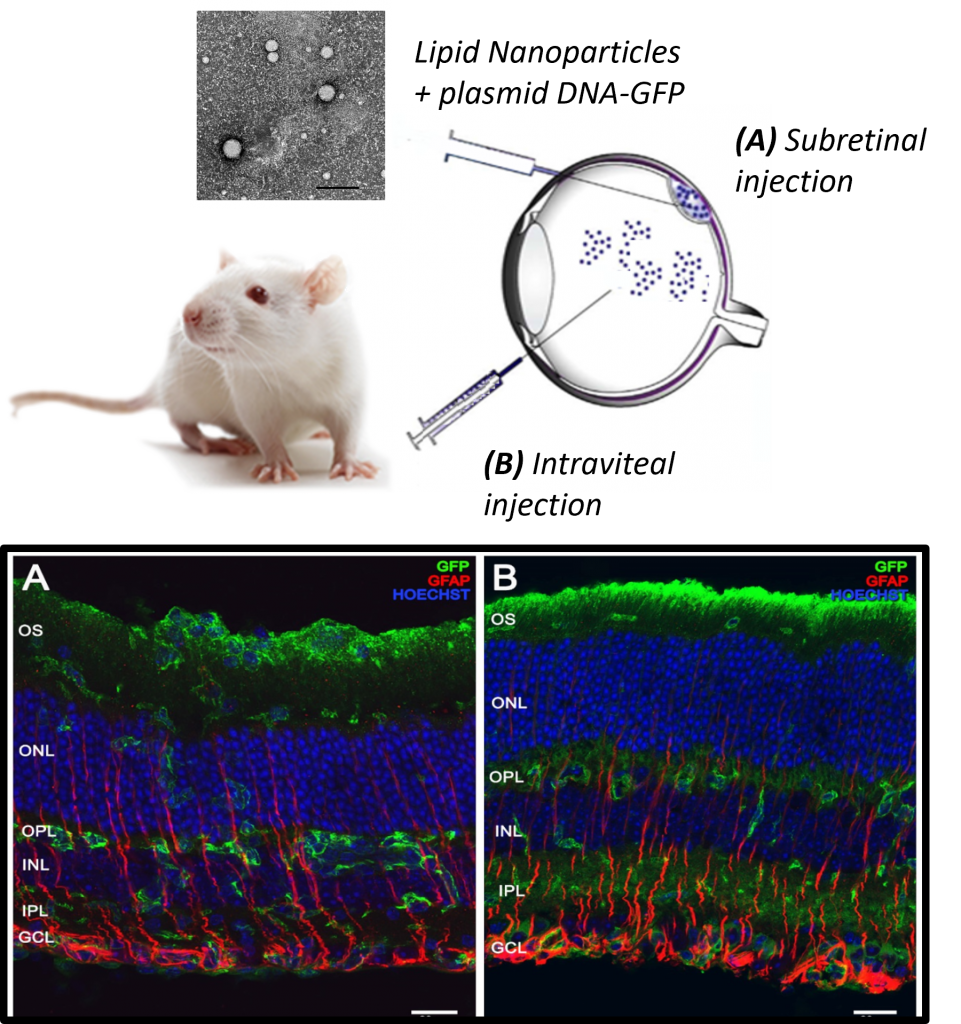
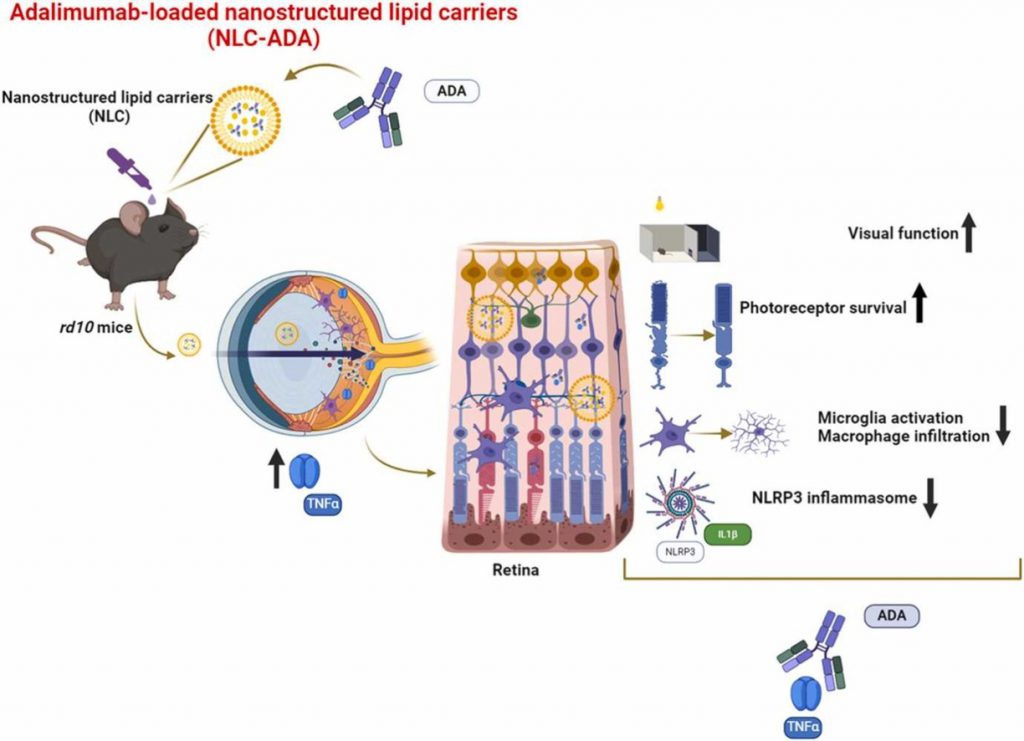
The new approach involves non-invasive eye drops containing adalimumab-loaded nanostructured lipid carriers (NLC–ADA). Adalimumab is a monoclonal antibody that targets TNFα, a pro-inflammatory cytokine involved in retinal inflammation and degeneration. Chronic inflammation has been increasingly recognized as a contributing factor in the progression of retinitis pigmentosa, despite the genetic basis of the disease.
In this preclinical study, researchers assessed the formulation’s physicochemical properties, retinal and corneal safety, and its ability to reduce inflammation and retinal degeneration. The results were highly encouraging: NLC–ADA eye drops improved retinal function, reduced retinal degeneration, and significantly decreased inflammatory markers, all without inducing toxicity.
This strategy offers a non-invasive route of administration and a new avenue for tackling retinitis pigmentosa
Regina Rodrigo
“This strategy offers a non-invasive route of administration and a new avenue for tackling retinitis pigmentosa,” said principal investigator Regina Rodrigo. “It is independent of the genetic defects and could be potentially useful for treating other inflammatory eye diseases.”
International patent application
Given the promising results, the participating institutions – CIPF, UPV/EHU, and CIBER – have jointly applied for an international patent, highlighting the global potential of this therapeutic innovation.
The study was supported by the Instituto de Salud Carlos III, the Generalitat Valenciana, and the ONCE Foundation, with the collaboration of the patient association RETINA Comunidad Valenciana.
For more information, the full article is available at:
Biomedicine & Pharmacotherapy, Volume 185 (2025)
Unit 10 of NANBIOSIS, led by Prof. José Luis Pedraz and Dr. Idoia Gallego, has contributed to this promising non-invasive therapy, aiding with its know-how in both the intellectual and technical element of the publication.
What is NANBIOSIS?
The goal of NANBIOSIS is to provide comprehensive and integrated advanced solutions for companies and research institutions in biomedical applications. All of this is done through a single-entry point, involving the design and production of biomaterials, nanomaterials, and their nanoconjugates. This includes their characterization from physical-chemical, functional, toxicological, and biological perspectives (preclinical validation).
In order to access our Cutting-Edge Biomedical Solutions with priority access, enter our Competitive Call here.
NANBIOSIS has worked with pharmaceutical companies of all sizes in the areas of drug delivery, biomaterials and regenerative medicine. Here are a few of them: