Impactful research with NANBIOSIS participation in the Poster Tour of CIBER-BBN & CIBEREHD Annual Conference.
2023 CIBER-BBN Annual meeting has taken place at Santemar Hotel, in Santander during November 6-7. This year the format of our annual conferences has been changed towards a collective event scheme between the CIBER-BBN and CIBEREHD thematic areas.
- On Monday 6 the scientific sessions werecommon for EHD and BBN, with appealing contents for the mixed audience.
- On Tuesday 7 EHD and BBN sessions will specific for each area in separate rooms (with common coffee break).
Posters of both areas were on display in the exhibit hall throughout the entirety of the Annual Meeting.
Moreover, at the “Posters & beers” session (Monday 6th: 6:00 p.m. – 7:00 p.m.) poster tours were organized where attendees could cast their vote for the best poster and use this one-on-one time with presenters to learn more, ask juicy questions and discuss their work. At 8:00 p.m., the awards ceremony took place for the best oral communication and best poster by young authors – for each area.

It was an impactful information sessions on research carried out by the groups of CIBER-BBN and CIBEREHD thematic areas.
The poster session is always a popular feature at CIBER-BBN Annual Meeting for acknowledgment NANBIOSIS units’ participation in the research carried out during the year. These are the works presented in 2023:
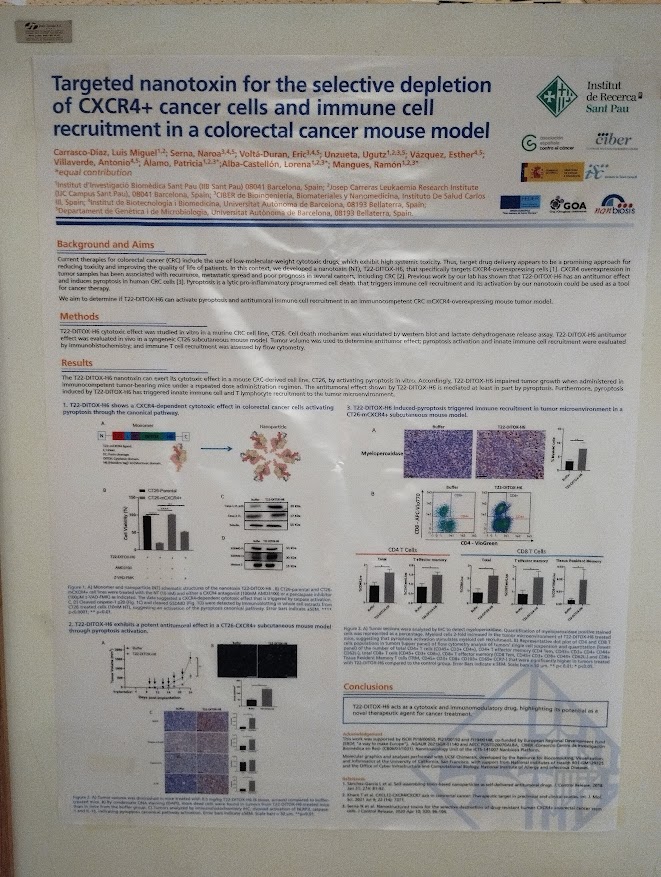
Targeted nanotoxin for the selective depletion of CXCR4+ cancer cells and immune cell recruitment in a colorectal cancer mouse model. Luis Miguel Carrasco-Díaz, Naroa Serna, Eric Voltà-Durán, Ugutz Unzueta, Esther Vázquez, Antonio Villaverde, Patricia Álamo, Lorena Alba-Castellón, Ramón Mangues. With participation of NANBIOSIS Units U1 Protein Production Platform (PPP) and U18 Nanotoxicology Unit . (Contact:
luismiguelcarrascodiaz@gmail.com)
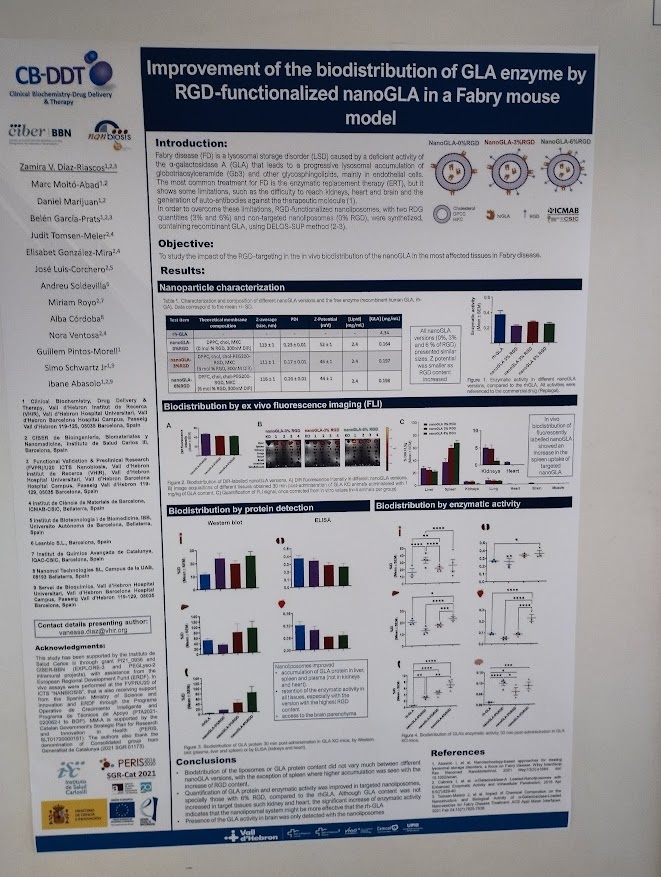
Improvement of the biodistribution of GLA enzyme by RGD-functionalized nanoGLA in a Fabry mouse model.
Zamira Vanessa Diaz Riascos, Marc Moltó Abad, Daniel Marijuan, Belen García Prats, Judit Tomsen Melero, Elisabet González Mira, Jose Luis Corchero, Andreu Soldevila, Miriam Royo, Alba Córdoba, Nora Ventosa, Guillem Pintos Morell, Simo Schwartz , Ibane Abasolo. With participation of the NANBIOSIS units U20 FVPR-In Vivo Experimental Platform, U3 Synthesis of Peptides Unit and U6 Biomaterial Processing and Nanostructuring Unit. (Contact:
vanessa.diaz@vhir.org)
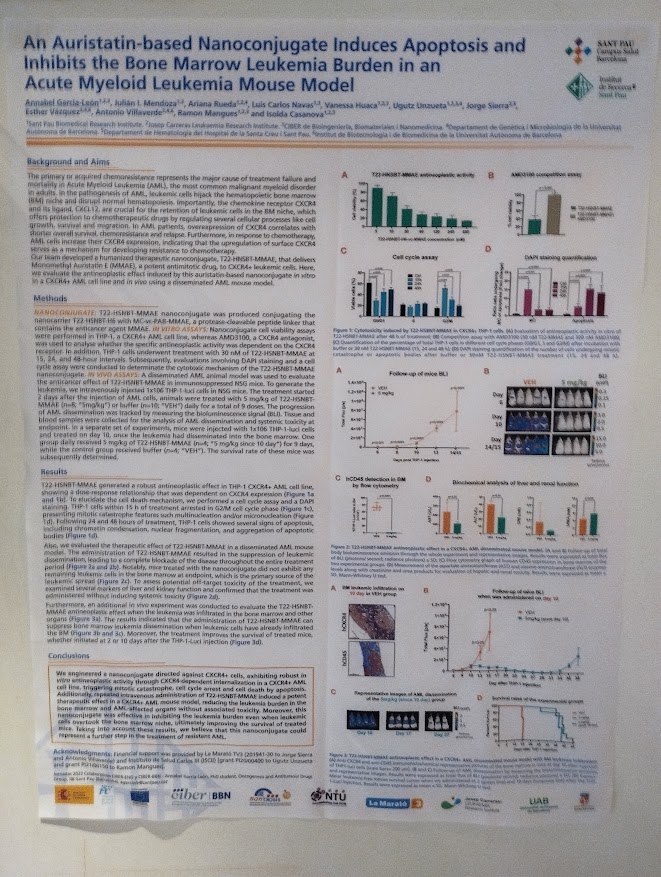
An auristatin-based nanoconjugate induces apoptosis and inhibits the bone marrow leukemia burden in an acute myeloid leukemia mouse model. Annabel Garcia-León, Julián I. Mendoza, Ariana Rueda, Luis Carlos Navas, Vanessa Huaca, Ugutz Unzueta, Jorge Sierra, Esther Vázquez, Antonio Villaverde, Ramon Mangues, Isolda Casanova. With participation of NANBIOSIS Units U1 Protein Production Platform (PPP) and U18 Nanotoxicology Unit. (Contact: agarciale@santpau.cat)
FVPR/U20-NANBIOSIS Service Platform: from the Synthesis and Characterization of Nanotechnology-based Therapies, to the in vitro and in vivo Preclinical Validation. Diana Rafael, Zamira V. Diaz Riascos, Belén García, Alejandra Palacios, Sandra Mancilla, Laura Garcia, Ibane Abasolo. Description of NANBIOSIS Unit 20 FVPR-In Vivo Experimental Platform. (Contact: diana.fernandes_de_so@vhir.org)

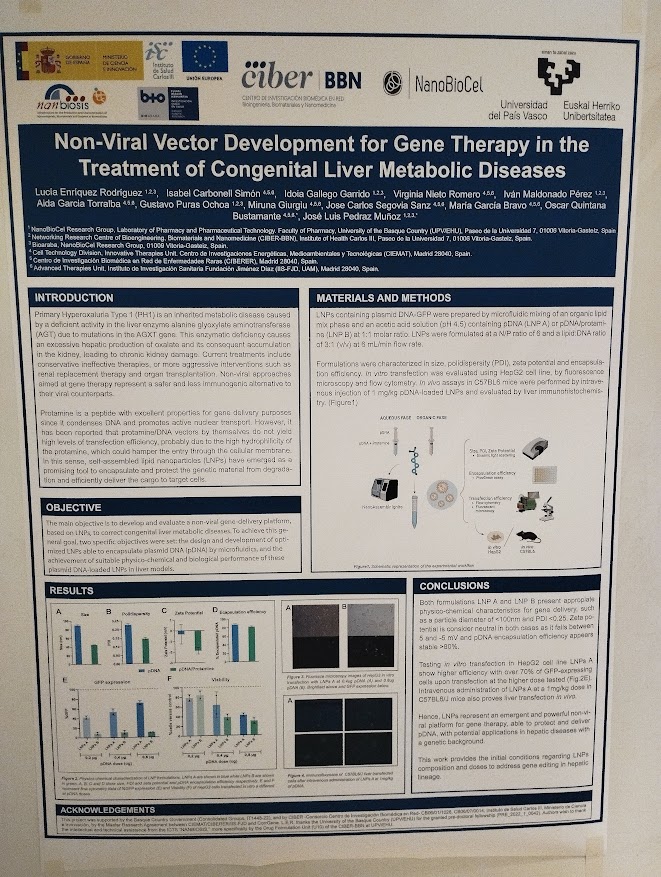
Non-Viral Vector Development for Gene Therapy in the Treatment of Congenital Liver Metabolic Diseases Lucía Enríquez Rodríguez, Isabel Carbonell Simón, Idoia Gallego Garrido, Virginia Nieto Romero, Iván Maldonado Pérez, Aida Garcia Torralba, Gustavo Puras Ochoa, Miruna Giurgiu, Jose Carlos Segovia Sanz, María García Bravo, Oscar Quintana Bustamante, José Luis Pedraz Muñoz. With participation of NANBIOSIS U10 Drug Formulation unit. (Contact: lucia.enriquez@ehu.eus)
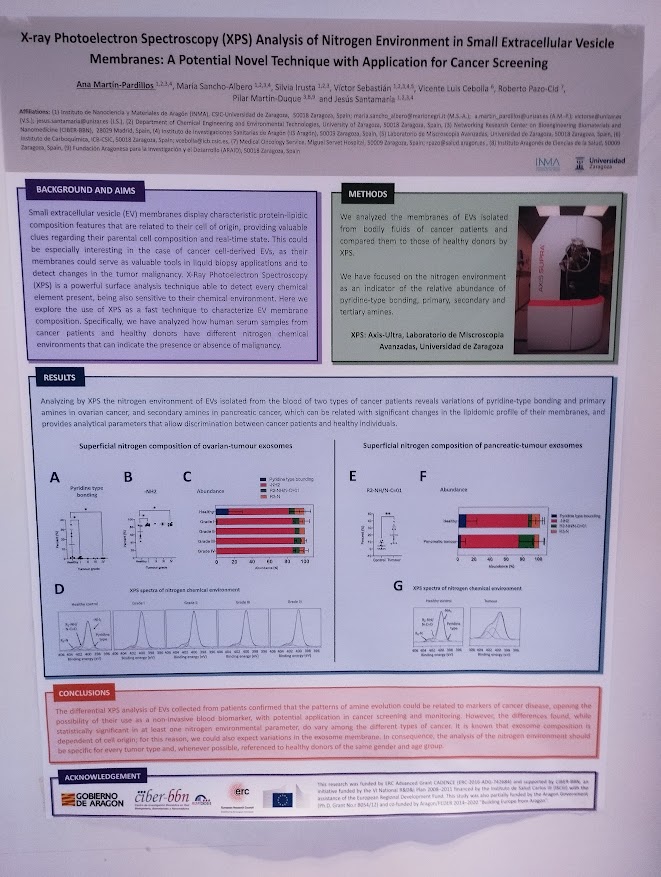
X-ray Photoelectron Spectroscopy (XPS) Analysis of Nitrogen Environment in Small Extracellular Vesicle Membranes: A Potential Novel Technique with Application for Cancer Screening.
Ana Martín-Pardillos, María Sancho-Albero , Silvia Irusta , Víctor Sebastián , Vicente Luis Cebolla , Roberto Pazo-Cid , Pilar Martín-Duque , Jesús Santamaría. With participation of NANBIOSIS U9 Synthesis of Nanoparticles Unit. (Contact: a.martin_pardillos@unizar.es)
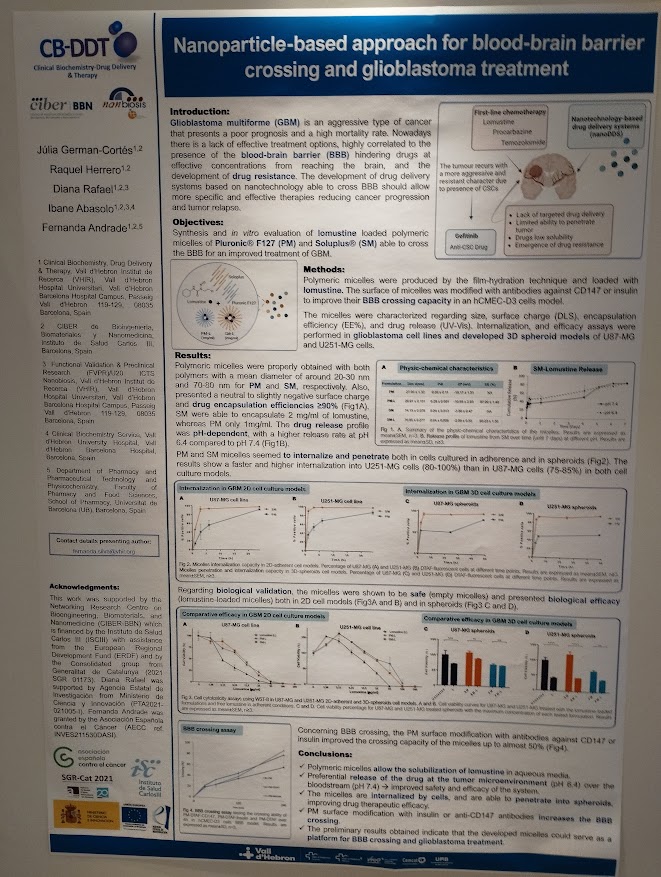
Nanoparticle-based approach for blood-brain-barrier crossing and glioblastoma treatment. Júlia German-Cortés, Raquel Herrero, Diana Rafael, Ibane Abasolo, Fernanda Andrade. With participation of NANBIOSIS Unit 20 FVPR-In Vivo Experimental Platform. (Contact: fernanda.silva@vhir.org)
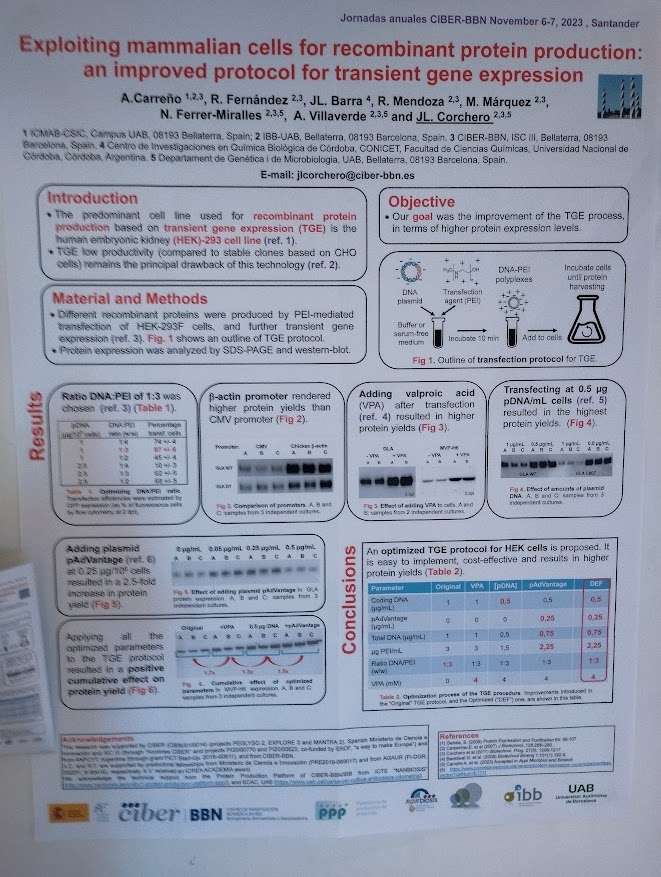
Exploiting mammalian cells for recombinant protein production: an improved protocol for transient gene expression. Aida Carreño Fibla, Roger Fernández Palomeras, José Luis Barra, Rosa Mendoza Moreno, Mercedes Márquez Martínez, Neus Ferrer-Miralles, Antonio Villaverde Corrales, José Luis Corchero Nieto. With participation of NANBIOSIS Units U1 Protein Production Platform (PPP). (Contact:jlcorchero@ciber-bbn.es)
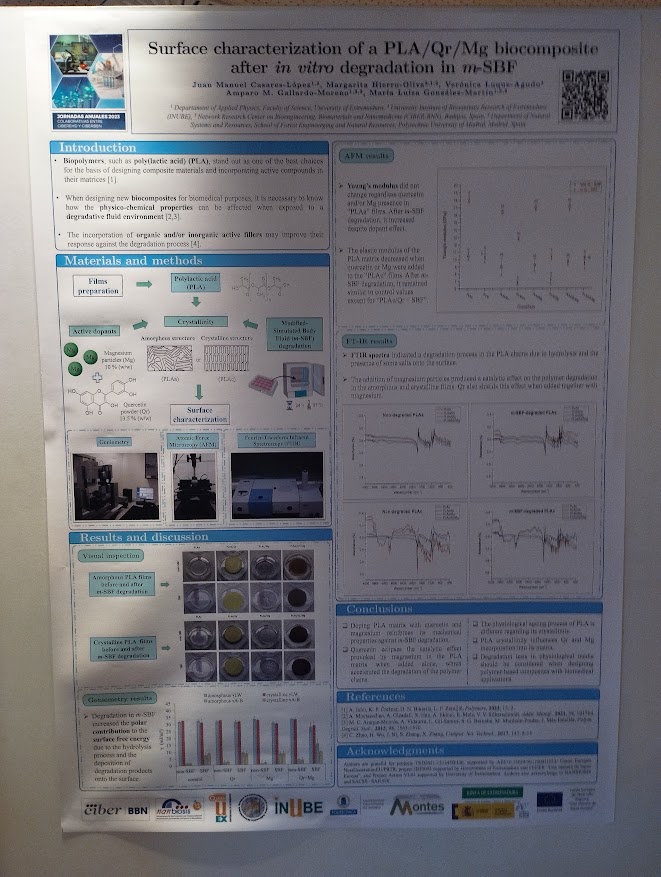
Surface characterization of a PLA/Qr/Mg biocomposite after in vitro degradation in m-SBF. Juan Manuel Casares-López, Margarita Hierro-Oliva, Verónica Luque-Agudo, Amparo M. Gallardo-Moreno, María Luisa González-Martín. With participation of Unit 16 Surface Characterization and Calorimetry Unit (Contact: mlglez@unex.es)
The poster session was an effective forum for the exchange of information and a means to communicate ideas
Related news: