A new pathway for the prevention of metastasis in colorectal cancer in humans is open: a nanomedicine that selectively eliminates metastatic stem cells
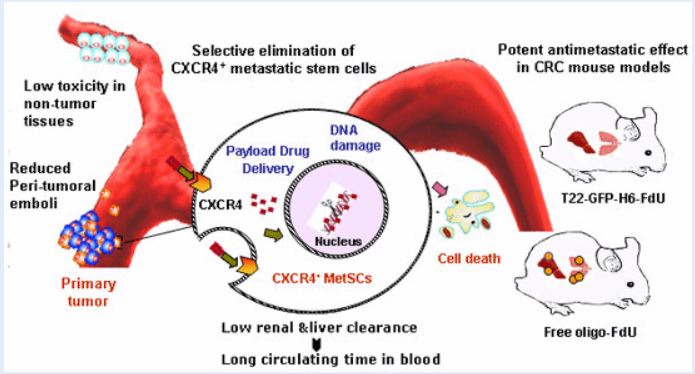
Researchers of NANBIOSIS U18 Nanotoxicology Unit and U1. Protein Production Platform (PPP) at the Biomedical Research Institute of Sant Pau (IIB Sant Pau), of the Hospital of Santa Creu i Sant Pau, of the Universitat Autònoma de Barcelona (UAB), the Superior Council of Scientific Research (CSIC) and the Center for Biomedical Research in Network (CIBER) have published an article in one of the most prestigious international scientific journals in the field of Molecular Medicine, EMBO Molecular Medicine. This article demonstrates the efficacy of the nanopharmaceutical that selectively removes metastatic stem cells in animal models of colon cancer. The new drug works like a drone that has a ligand that identifies a receptor (CXCR4) in the metastatic stem cells, administers the drug and destroys these cells by blocking the metastasis. The drug acts only on metastatic tumor cells and not on healthy cells, so it avoids the general toxicity associated with the usual treatments
This nanopharmaceutical has been successfully tested in animal models of colorectal cancer, but could be used in 20 additional tumor types that express CXCR4,
as in those of prostate, breast, ovary and others
This is the first drug in the world selectively antimetastatic that addresses the medical need to block metastatic spread, the main cause of death in cancer patients, while eliminating the toxicity and adverse effects of conventional treatments
The Hospital de Sant Pau could be the first center in the world to carry out clinical trials that evaluate this new drug in patients, prior to its possible introduction in clinical therapeutics
It has been observed that this receptor is overexpressed in at least 20 different types of cancer, including those of the prostate, breast, ovary and others not as common as the pancreas. This means that this nanoparticle can be targeted to treat different types of neoplasms, making it a very versatile vehicle that can transport different therapeutic molecules of high potency.
Article of reference:
, , , , , , , , , ,, , , Selective depletion of metastatic stem cells as therapy for human colorectal cancer. EMBO Molecular Medicine DOI 10.15252/emmm.201708772
The team of researchers, led by Dr. Ramón Magues de l’IIB Sant Pau, Prof. Antonio Villaverde of the UAB and Dr. Esther Vázquez of the UAB, have shown that the drug acts only on metastasis-initiating cells through its specific interaction between a peptide present in the protein nanoparticle that transports it and the cellular receptor CXCR4 that is overexpressed in tumor cells. This allows attacking only the tumor cells, blocking their dissemination in early stages, in a way that prevents the appearance of metastasis while avoiding the adverse effects derived from the usual treatments.
Nanoligent, a new spin-off to finance the nanoparticle
In June 2017, researchers from the IIB Sant Pau, from the Institute of Biotechnology and Biomedicine of the UAB and the CIBER from Bioengineering, Biomaterials and Nanomedicine (CIBER-BBN) who signed the article published now in EMBO Molecular Medicine created a spin-off, Nanoligent , with the aspiration to develop the first drug designed to eliminate metastatic cells.
This company, which has more than 10 years of studies behind it, is directed by Dr. Manuel Rodríguez Mariscal, a professional with a long experience in the field of investment and the creation of biotechnology companies and aims to obtain financing for the realization of the project.