Advances in MRI and Brain Tumor Imaging: NANBIOSIS at ISMRM 2025
NANBIOSIS Unit 25 showcased preclinical MRI advances at ISMRM 2025 Iberian Chapter with talks, posters, and international collaboration.
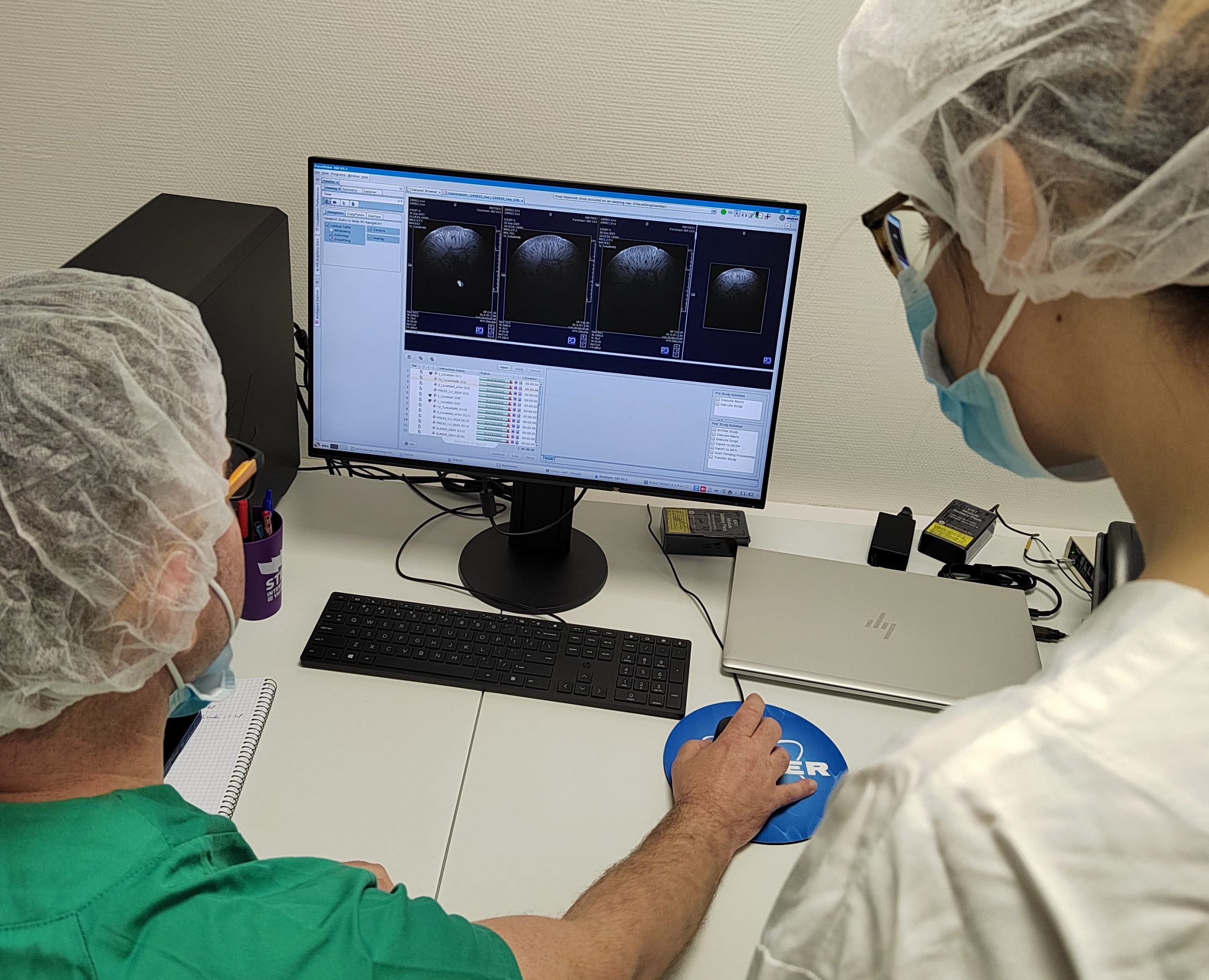
Barcelona, July 8, 2025 — NANBIOSIS Unit 25: NMR: Biomedical Applications I, played a leading role at the 5th Annual Meeting of the International Society for Magnetic Resonance in Medicine (ISMRM), Iberian Chapter, held on July 3–4 at the Institute for Bioengineering of Catalonia (IBEC). The event brought together top researchers in Magnetic Resonance Imaging (MRI) and Nuclear Magnetic Resonance (NMR) from Spain and Portugal, highlighting cutting-edge applications in preclinical imaging and biomedical research.
Strong Scientific and Organizational Presence from NANBIOSIS
Researchers from Unit 25 of NANBIOSIS, a key platform for NMR-based biomedical applications at the Universitat Autònoma de Barcelona (UAB), made major contributions to the scientific program. Dr. Ana Paula Candiota, Scientific Director, and Dr. Silvia Lope-Piedrafita, Scientific Coordinator, were both members of the local organizing committee and actively participated in the scientific sessions.

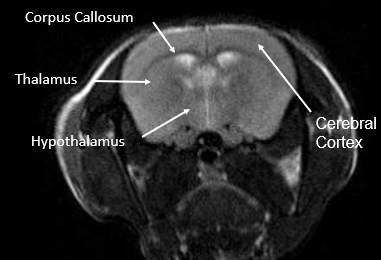
Dr. Lope-Piedrafita gave an oral presentation titled “Applying longitudinal MRI for tumor evaluation in two immunocompetent chicken chorioallantoic membrane (CAM) cancer xenograft models,” showcasing innovative imaging approaches in oncology research. The study was co-authored by Dr. Candiota.
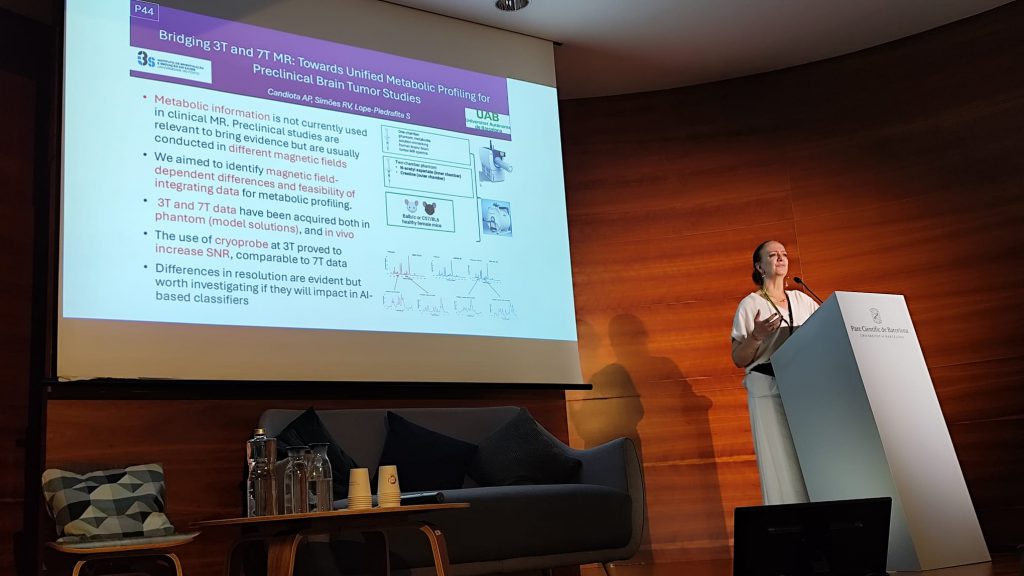
Dr. Candiota also delivered three poster pitches, including “Bridging 3T and 7T MR: Towards Unified Metabolic Profiling for Preclinical Brain Tumor Studies.” Part of this research was conducted during her CIBER-funded scientific mobility stay in Portugal, reflecting ongoing international collaboration within the network.
About Unit 25: Advanced NMR Tools for Biomedical Applications
Unit 25 of NANBIOSIS is a unique research infrastructure offering in vivo, ex vivo, and in vitro NMR services. It is jointly operated by the Nuclear Magnetic Resonance Facility (SeRMN) and the Institute of Biotechnology and Biomedicine (IBB) at UAB, and coordinated by Dr. Candiota herself.
This infrastructure enables high-resolution molecular imaging, metabolic profiling, and biomarker discovery for translational research in oncology, neurology, and other biomedical fields. For more information about the Unit, you can visit the portfolio here.
Promoting Innovation in Magnetic Resonance Imaging
The participation of NANBIOSIS U25 in the ISMRM 2025 Iberian Chapter meeting reinforces its role as a national and international reference in the development and application of NMR and MRI technologies for biomedical research and preclinical trials. For further details about the event, visit the official ISMRM Iberian Chapter site.
What is NANBIOSIS?
The goal of NANBIOSIS is to provide comprehensive and integrated advanced solutions for companies and research institutions in biomedical applications. All of this is done through a single-entry point, involving the design and production of biomaterials, nanomaterials, and their nanoconjugates. This includes their characterization from physical-chemical, functional, toxicological, and biological perspectives (preclinical validation).
In order to access our Cutting-Edge Biomedical Solutions with priority access, enter our Competitive Call here.
NANBIOSIS has worked with pharmaceutical companies of all sizes in the areas of drug delivery, biomaterials and regenerative medicine. Here are a few of them: