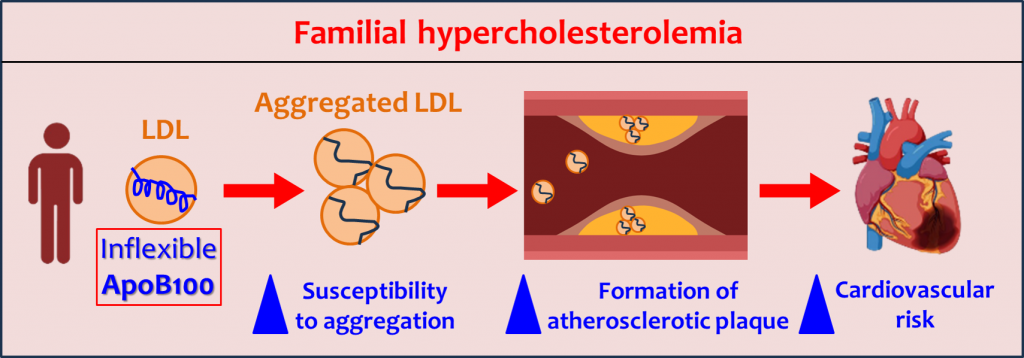
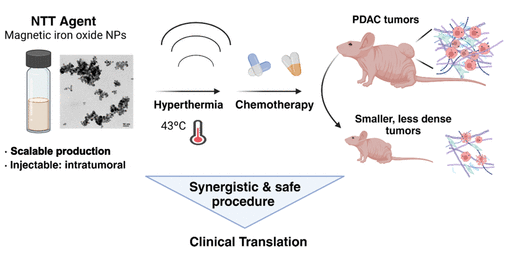
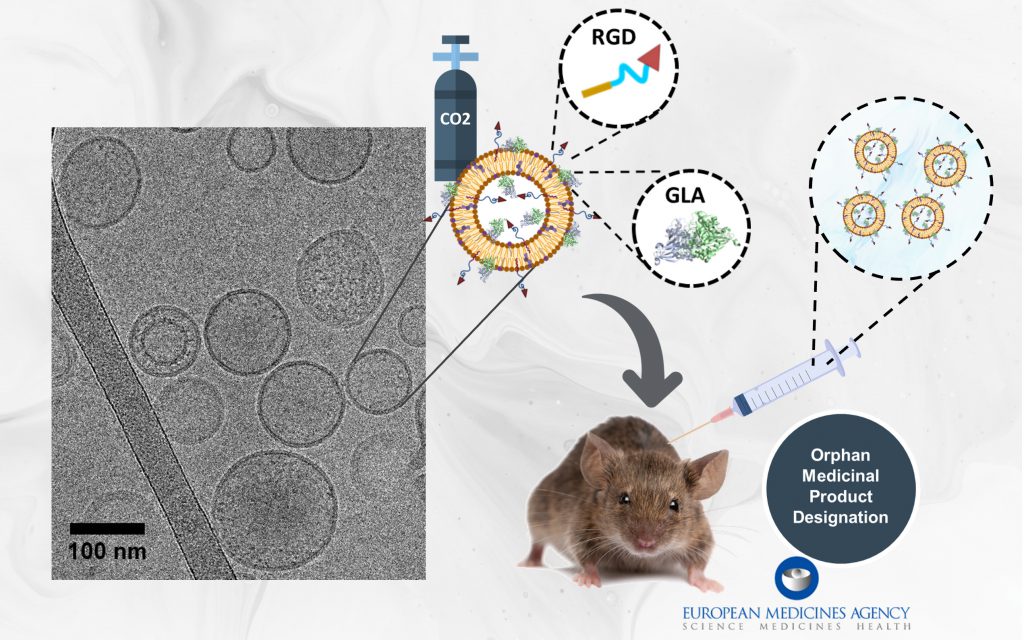
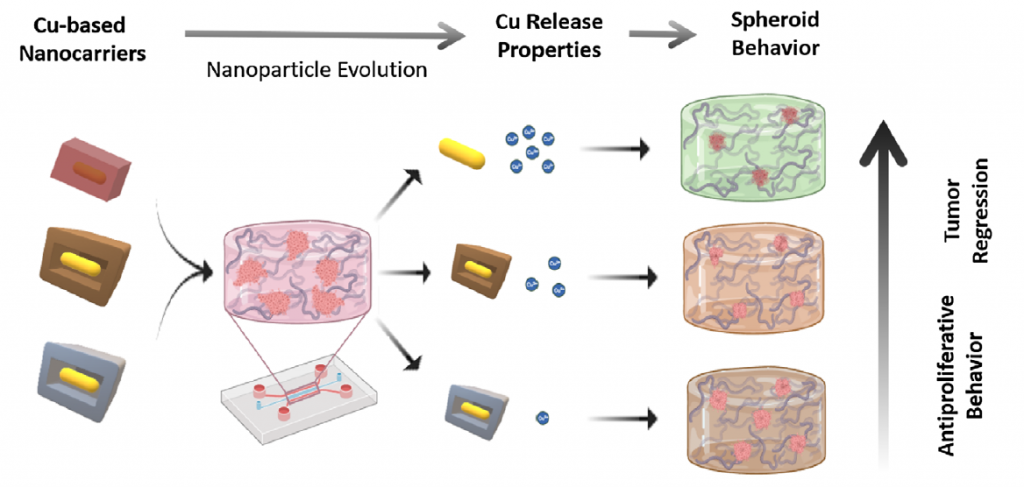
Spain-Portugal Collaboration Advances New Preclinical MRI and Machine Learning Research
Dr. Candiota’s visit to I3S strengthens Spain-Portugal collaboration in preclinical MRI, advancing data compatibility and future machine learning studies.
Barcelona-Porto, February 2025. Dr. Ana Paula Candiota, Scientific Director of Unit 25 of NANBIOSIS, is currently undertaking a short scientific stage at the Preclinical MRI Lab of the Instituto de Investigação e Inovação em Saúde (I3S) at the University of Porto. This initiative highlights the strong international collaboration between Spanish and Portuguese research institutions and is supported by a mobility call from CIBER-BBN.
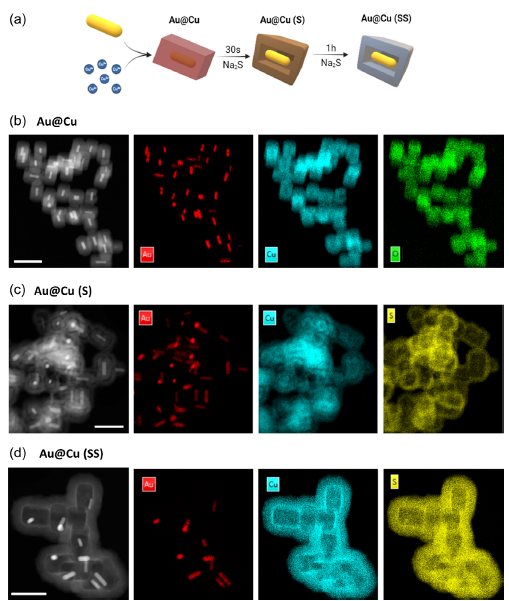
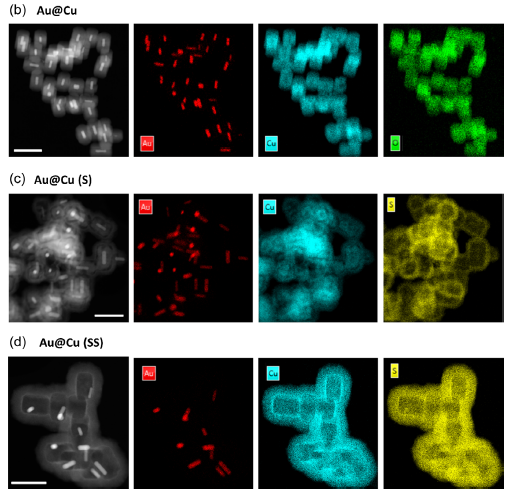
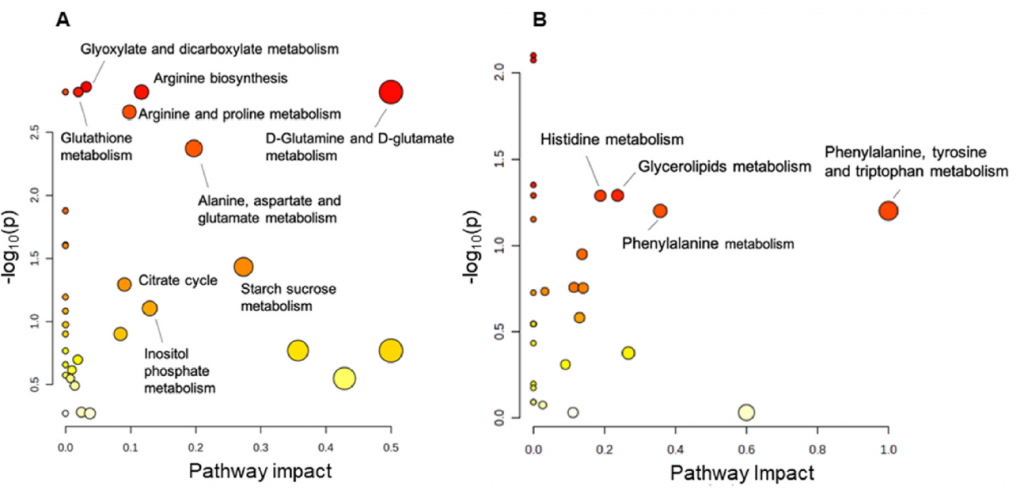
Dr. Candiota is working alongside Dr. Rui Simões, the current head of the I3S Preclinical MRI Lab and a former member of her research group from 2006 to 2010. Their joint efforts focus on evaluating the compatibility of magnetic resonance (MR) data acquired at different magnetic fields (3T and 7T) and under different Bruker Paravision versions. This work is crucial for integrating data in future studies utilizing machine learning approaches. Complementing this research, the 7T MR dataset will be acquired and completed by the Scientific Coordinator of Unit 25, Dr. Silvia Lope, at our facilities in Universitat Autònoma de Barcelona (UAB).
Insternational Collaboration to strengthen NANBIOSIS
This collaboration also plays a strategic role in the ongoing upgrade of the preclinical MR scanner of Unit 25. Notably, the 3T MR equipment at I3S operates under the same Bruker Paravision version that will soon be installed at the NABIOSIS Unit. Dr. Candiota’s stay in Porto will facilitate her familiarization with the new console’s capabilities, ensuring a smoother transition and enhanced research potential at NANBIOSIS.

As part of this international exchange, Dr. Candiota has been invited to deliver a seminar at I3S, fostering further collaborative opportunities between Spain and Portugal in preclinical imaging research. This initiative underscores the importance of cross-border scientific cooperation in advancing biomedical research and technological innovation.
What is NANBIOSIS?
The goal of NANBIOSIS is to provide comprehensive and integrated advanced solutions for companies and research institutions in biomedical applications. All of this is done through a single-entry point, involving the design and production of biomaterials, nanomaterials, and their nanoconjugates. This includes their characterization from physical-chemical, functional, toxicological, and biological perspectives (preclinical validation).
In order to access our Cutting-Edge Biomedical Solutions with priority access, enter our Competitive Call here.
NANBIOSIS has worked with pharmaceutical companies of all sizes in the areas of drug delivery, biomaterials and regenerative medicine. Here are a few of them: