AKT2 as a promising target for future anti-cancer therapies
The researchers of NANBIOSIS U20, led by Ibane Abásolo and Simó Schwartz have published a new article on the scientific magazine Cancers, with the title Pivotal Role of AKT2 during Dynamic Phenotypic Change of Breast Cancer Stem Cells
All the in vivo studies were performed by NANBIOSIS U20 In Vivo Experimental Platform.
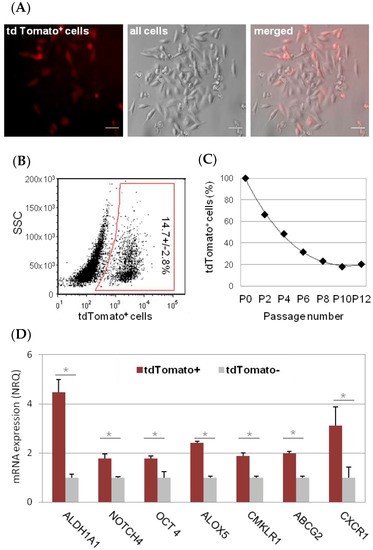
Therapeutic resistance seen in aggressive forms of breast cancer remains challenging for current treatments. More than half of the patients suffer from a disease relapse, most of them with distant metastases. Cancer maintenance, resistance to therapy, and metastatic disease seem to be sustained by the presence of cancer stem cells (CSC) within a tumor. The difficulty in targeting this subpopulation derives from their dynamic interconversion process, where CSC can differentiate to non-CSC, which in turn de-differentiate into cells with CSC properties. Using fluorescent CSC models driven by the expression of ALDH1A 1(aldehyde dehydrogenase 1A1), we confirmed this dynamic phenotypic change in MDA-MB-231 breast cancer cells and to identify Serine/Threonine Kinase 2 (AKT2) as an important player in the process. To confirm the central role of AKT2, we silenced AKT2 expression via small interfering RNA and using a chemical inhibitor (CCT128930), in both CSC and non-CSC from different cancer cell lines. Our results revealed that AKT2 inhibition effectively prevents non-CSC reversion through mesenchymal to epithelial transition, reducing invasion and colony formation ability of both, non-CSC and CSC. Further, AKT2 inhibition reduced CSC survival in low attachment conditions. Interestingly, in orthotopic tumor mouse models, high expression levels of AKT2 were detected in circulating tumor cells (CTC). These findings suggest AKT2 as a promising target for future anti-cancer therapies at three important levels: (i) Epithelial-to-mesenchymal transition (EMT) reversion and maintenance of CSC subpopulation in primary tumors, (ii) reduction of CTC and the likelihood of metastatic spread, and (iii) prevention of tumor recurrence through inhibition of CSC tumorigenic and metastatic potentia