NANBIOSIS joins pioneering research on the permeability of epithelia for new Drug Delivery
NANBIOSIS Unit 1 supports study on c-CPE protein to modulate epithelial permeability, enabling safer, targeted drug delivery.
Barcelona, april 2025. Researchers from the Institute of Biotechnology and Biomedicine (IBB) at the Universitat Autònoma de Barcelona (UAB) and other institutions have published a new study in Molecular Pharmaceutics demonstrating the dual behavior of a fragment of a bacterial toxin in modulating epithelial permeability. Thus providing with a key factor for improving drug delivery through epithelial barriers.
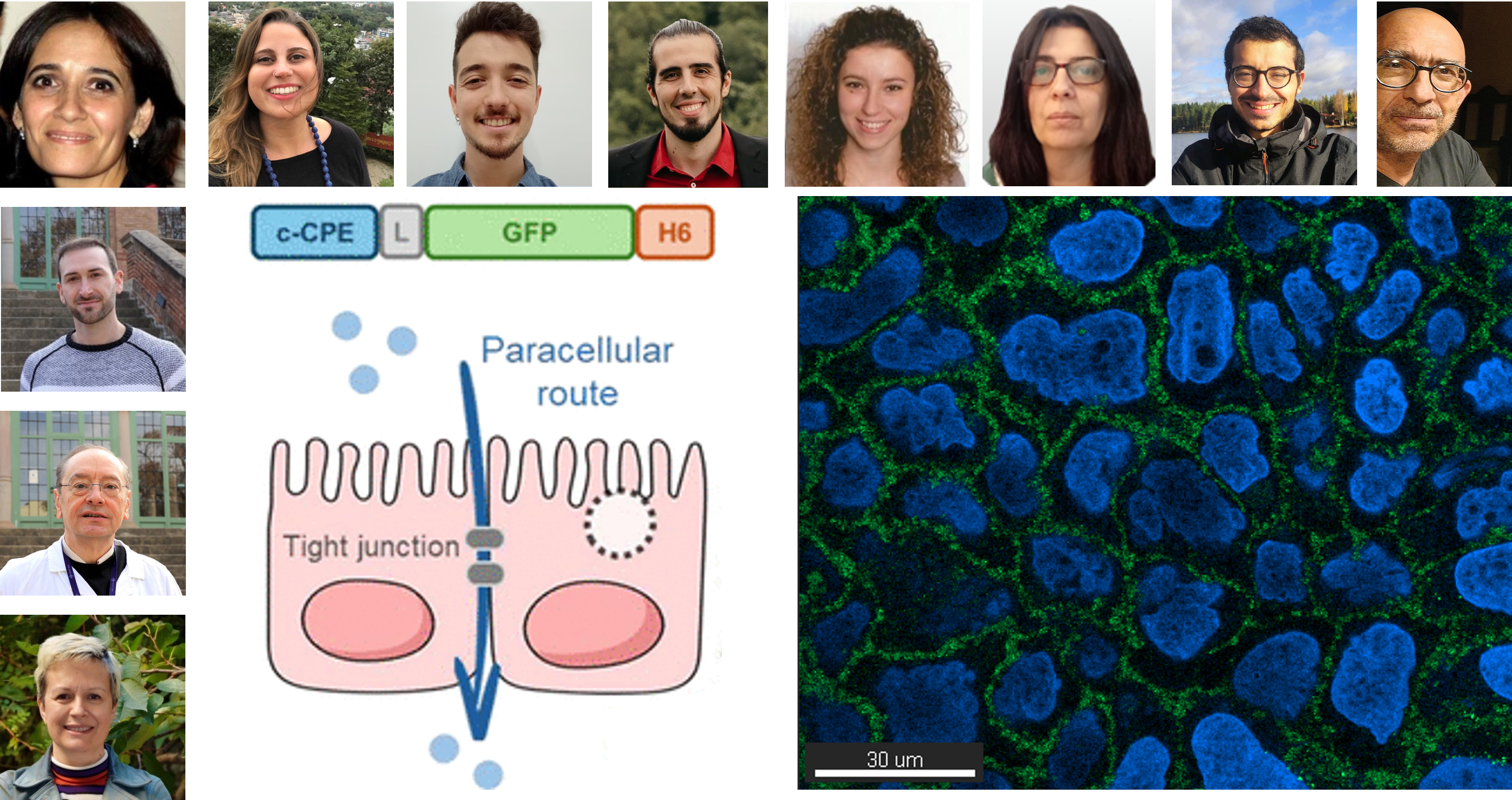
The study, titled “Trans-Mediated, Cis-Inhibited Paradoxical Activity of Clostridium perfringens Enterotoxin (c-CPE) in Modulating Epithelial Permeability” (DOI: 10.1021/acs.molpharmaceut.4c01205), explores how the c-CPE domain of Clostridium perfringens enterotoxin affects tight junctions in epithelial cell monolayers.
Using Caco-2 cell cultures, the researchers have shown that fusion proteins containing the c-CPE fragment can bind to epithelial cells without triggering internalization or cytotoxic effects, and importantly, they facilitate the passage of other therapeutic proteins across epithelial barriers. Interestingly, these c-CPE-tagged proteins remain trapped at the tight junctions and cannot cross the barrier themselves, likely due to their high binding affinity.
This finding highlights a novel and practical strategy: using c-CPE as a temporary gate-opener to enhance drug or biologic delivery across epithelial tissues, without the need for chemical disruption—an approach with promising implications for drug development and targeted delivery platforms.
This paradoxical behavior offers valuable insights for drug delivery strategies that aim to temporarily and selectively open epithelial barriers, such as those of the intestine or respiratory tract, to facilitate the absorption of therapeutic macromolecules.
Scientific collaboration for complex drug delivery
The research was conducted by scientists from the Institut de Biotecnologia i de Biomedicina (IBB-UAB), the Departament de Genètica i de Microbiologia at the UAB, as well as the Centro de Investigación Biomédica en Red de Bioingeniería, Biomateriales y Nanomedicina (CIBER-BBN) from Instituto de Salud Carlos III.
NANBIOSIS Unit 1 – Protein Production Platform (PPP), led by Prof. Antonio Villaverde and located at the IBB-UAB, supported this research by participating in the production of the recombinant c-CPE-containing protein used in the experiments. The platform specializes in the design, expression, and purification of recombinant proteins for biomedical research and plays a central role in enabling access to high-quality protein biotechnologies for both academic and industrial partners.
This collaboration underscores the importance of advanced research infrastructures like NANBIOSIS in facilitating innovative biomedical research.

What is NANBIOSIS?
The goal of NANBIOSIS is to provide comprehensive and integrated advanced solutions for companies and research institutions in biomedical applications. All of this is done through a single-entry point, involving the design and production of biomaterials, nanomaterials, and their nanoconjugates. This includes their characterization from physical-chemical, functional, toxicological, and biological perspectives (preclinical validation).
In order to access our Cutting-Edge Biomedical Solutions with priority access, enter our Competitive Call here.
NANBIOSIS has worked with pharmaceutical companies of all sizes in the areas of drug delivery, biomaterials and regenerative medicine. Here are a few of them: