U11-E08. Thermohygrometer
Thermohygrometer TESTO 175-H2 38244023/008 (0 to 50) ªC // (10 to 75) %HR 0,1 ºC // %HR
Thermohygrometer TESTO 175-H2 38244023/008 (0 to 50) ªC // (10 to 75) %HR 0,1 ºC // %HR
The pharmaceutical formulation laboratory is enabled and equipped to store, formulate and reconstitute trial products for differents in vivo preclinical studies with strict quality standards. In addition to drug formulation for preclinical studies and its use in experimental animals. This formulation laboratory equipped to formulate, reconstitute and guarantee the correct storage of the test products used in studies.
Customer benefits
The laboratories include in the unit are certified with ISO-9001 and Good Laboratory Practices (GLP), strict quality standards that allow the production of high-precision results
Also, the studies can be performed under regulatory requirements too, since the performing institution is Certified for Good Laboratory Practices.
Therefore, preclinical studies and safety and efficacy studies in animal models can be carried out in compliance with the strict guidelines of regulatory agencies, ensuring the reliability and traceability of all results and tests carried out in their different services.
Target customer
The services offered in this unit may be of interest to different companies and laboratories that work within the pharmaceutical industry. Companies whose objective is to test possible candidates for molecules, drugs or medical devices. Both in previous legal preclinical studies and animal models of specific pathologies.
References
Cell Culture Laboratory
• Flow cytometer
• Dripper electrostatic
• Autoclave
• Fluorescence inverted microscope imaging system
• Inverted microscope with imaging system
• system for processing micro and nanoparticles by atomization
• Spray-dryer
• High pressure homogenizer
• Freezedryer
• Particle size analyzer in solid
• Coulter Particle size analyzer
• Particle size analyzer and Zetasizer
• Compression Tablet machines (eccentric and rotary)
• Fluid Bed
• coating machines
• Capsules machine
• Blister packing machine
• One step mixer Granulator
• Liquid chromatograph with fluorescence detection
• Liquid chromatographs with UV detection
• Liquid chromatograph with electrochemical detection
• HPLC-MS
• HPLC-MS/MS
• UPLC
• GC-MS, GC-NPD
• Dissolution test Equipment
• Desintegration tester
• Friabilometer
• Durometer
• determination of moisture equipment
• Viscometer
• -80 º C Freezers
• Climate chambers with temperature and humidity control
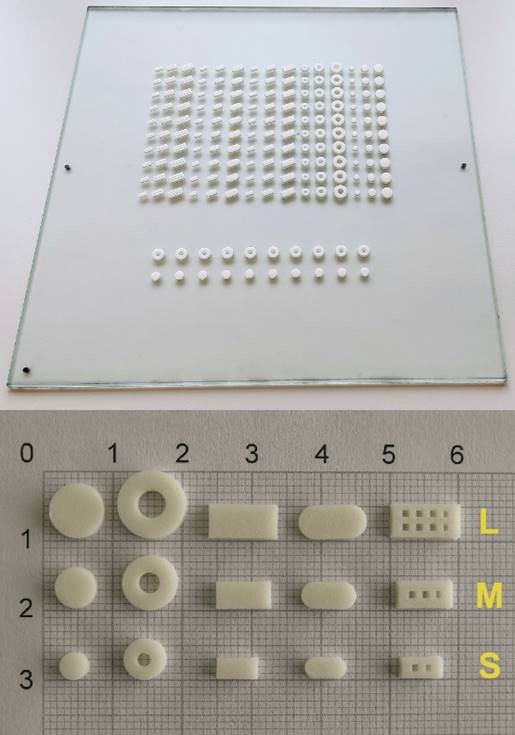
Classic parenteral dosage forms: solutions, suspensions, emulsions,…
Parenteral dosage forms for sustained release.
Topical dossage: creams, gels,…
Classic oral dosage forms: capsules, tablets, coated tablets, microgranules,…
Oral dossages forms for suistanable release: hidrophylic matrixes, lipid matrixes, pellets,…
The techniques we use to make the pharmaceutical dosage forms are:
Customer benefits
Target customer
Additional information
The following is an example of our bioprinted materials, which were used in the first study on 3D screen printing in the fabrication of drug delivery systems:
Reference: D. Moldenhauer, et al. 3D screen printing – An innovative technology for large-scale manufacturing of pharmaceutical dosage forms. International Journal of Pharmaceutics, Vol. 592, 2021, 120096, ISSN 0378-5173, DOI: 10.1016/j.ijpharm.2020.120096.
Design & Development of micro- & nanocapsules for conventional drugs, peptides and proteins.
Customer benefits
Use of micro- and nanotechnologies for the design of specific formulations and capsules with conventional drugs, peptides and proteins for preclinical evaluation.
Target customer
Design & Development of lipid nanoparticles to encapsulate therapeutic actives for drug delivery or gene delivery purposes: SLNs (solid lipid nanoparticles), NLCs (nanostructured lipid carriers), LNPs (self-assembled lipid nanoparticles by microfluidic technology) and niosomes.
Customer benefits
Customized elaboration of nanovehicles to deliver the therapeutic material of interest (mRNA, plasmid DNA, drugs, growth factors, peptides, antibodies…). We are specialists in designing and developing formulations for several therapeutic purposes. Among them, we develop self-assembled lipid nanoparticles, which are formed by fast microfluidic mixing in a NanoAssemblr® platform. These kinds of nanoparticles have emerged as an ideal nanotechnology approach for drug delivery and non-viral gene therapy by the high efficient encapsulation and protection of the therapeutic material from degradation. This microfluidic technology, that has been employed for COVID-19 vaccine, works under GMP conditions and enables reproducibility and scalability, which enhances its clinical translation.
Physicochemical characterization of nanovehicles, following SOPs, regarding mean particle size, polydispersity index and zeta potential. Additional parameters can be determined, such as, pH of nanoparticle suspension, microscopic morphology of the nanoparticles, encapsulation efficiency of the active principle and in vitro release profile. Optionally, biological evaluation can be performed, such as transfection efficiency analysis of non-viral nanovehicles and/or cytotoxicity assays following SOPs that include the ISO 10993-5-2019 Biological evaluation of medical devices.
Target customer
References
Additional information
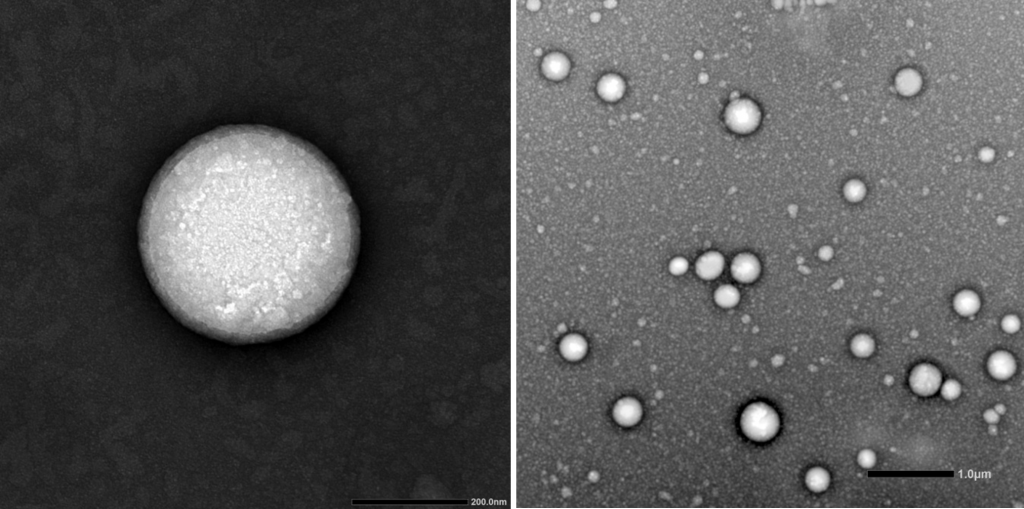
Cell encapsulation in alginate based microcapsules.
Optionally, cell viability assays, metabolic activity and other phenotypic / biological studies can be carried out.
Customer benefits
Microencapsulation of cells allows their transplantation in absence of immunosuppression along a wide variety of diseases, with long retention in the engrafted tissue. This strategy improves the engraftment rate and survival of transplanted cells following implantation.
Target customer
Preclinical use for in vivo models
References
Additional information
Microencapsulated cardiospheres 10X Captured Brightfield with DM_RGB_Brightfield with DM.
Reactor for laser-induced pyrolysis for controlled synthesis of nanoparticles from vapor phase precursors, aerosols and vaporized mixtures of gases and liquids. This reactor is composed of the following components:
•• Infrared CO2 laser resonator.
•• RF and DC three-phase radiofrequency supply at 360 and 440 V.
•• External cooler unit.
•• Laser power meter Unit by Ophir Optronics Ltd.
•• Reaction chamber for working under vacuum (10-6 mbar).
•• System for mixing and vaporization of gases and liquids to produce aerosol and gas mixtures at known compositions (Brooks Instrument).
System for measuring of adsorption and drugs delivery consisting of an UV -Visible spectrometer (Agilent) with flow cell and diode array detector (DAD ).
