Researchers from NANBIOSIS-CIBER-BBN have developed a new type of protein biomaterial that allows a continuous release over time of therapeutic proteins when administered subcutaneously in laboratory animals.
These results are the result of the stable scientific collaboration between the researchers of NANBIOSIS Units 1 Protein Production Platform (PPP)and 18 Nanotoxicology Unit, led by Toni Villaverde and Ramón Mangues at the Institute of Biotechnology and Biomedicine of the Autonomous University of Barcelona (IBB-UAB) and the Institut About the Hospital de Sant Pau and has had the participation of the Institute of Biological and Technological Research of the National University of Córdoba-CONICET, in Argentina
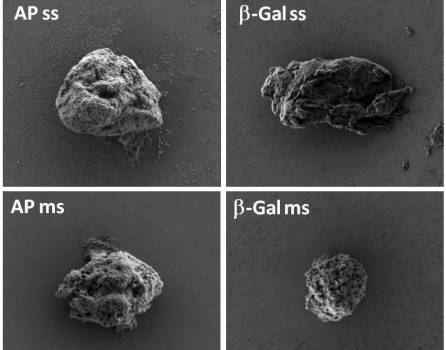
“These structures, of a few micrometers in diameter, contain functional proteins that are released in a manner similar to the release of human hormones in the endocrine system,” says Antonio Villaverde. Ramón Mangues explains that “the new biomaterial mimics a common bacterial product in biotechnological processes called ‘inclusion bodies’, of pharmacological interest, which in this artificial version offers a wide range of therapeutic possibilities in the field of oncology and in any other field clinic that requires sustained release over time.” Researchers have used common enzymes in biotechnology as a model and a nanostructured bacterial toxin that targets metastatic cells of human colorectal cancer, which has been tested in animal models. “In this way we have managed to generate both immobilized catalysts and a new long-acting anti-tumor drug,” said the researchers responsible for the research.
The developed artificial protein granules, which had previously been proposed as ‘nanopills’ (tablets of therapeutic material on a nanoscopic scale), mimic bacterial inclusion bodies and offer enormous clinical potential in the field of vaccinology and as release systems Drug controlled.
“We have seen that natural inclusion bodies, administered as medicines, can generate unwanted immune responses due to the inevitable contamination with bacterial materials,” the researchers comment. However, in the new work, the development of artificial inclusion bodies with secretion capacity “avoids many of the regulatory problems associated with the potential development of bacterial nanopills, and offers a cross platform for obtaining functional components in cosmetics and in clinic” they add.
This work points to artificial inclusion bodies as a new exploitable category of biomaterials for biotechnological applications with a more simple manufacturing and clinical applications.
Reference article:
Julieta M. Sánchez, Hèctor López ‐ Laguna, Patricia Álamo, Naroa Serna, Alejandro Sánchez ‐ Chardi, Verónica Nolan, Olivia Cano ‐ Garrido, Isolda Casanova, Ugutz Unzueta, Esther Vazquez, Ramon Mangues, Antonio Villaverde Artificial Inclusion Bodies for Clinical Development
https: //doi.org/10.1002/advs.201902420








