
Design advanced antimicrobial nanoparticles for comprehensive, affordable wound treatment, addressing multiple aspects of infection with enhanced effectiveness.
Scientific Leader / leaders of the project: Nora Ventosa / Maria Luisa González
Coordinator of the project: Guillem Vargas / Margarita Hierro
There are several antimicrobial materials on the market to prevent or treat microbial infections. Despite the large number of strategies developed in recent decades, there is still a need for effective and affordable treatments for infected wounds that can address more than one aspect of antimicrobial treatment. Nanoparticles which can have intrinsic antimicrobial activity, are good candidates for this purpose. In addition, these nanoparticles can enhance their antimicrobial activity by loading hydrophilic or hydrophobic molecules with antimicrobial properties.
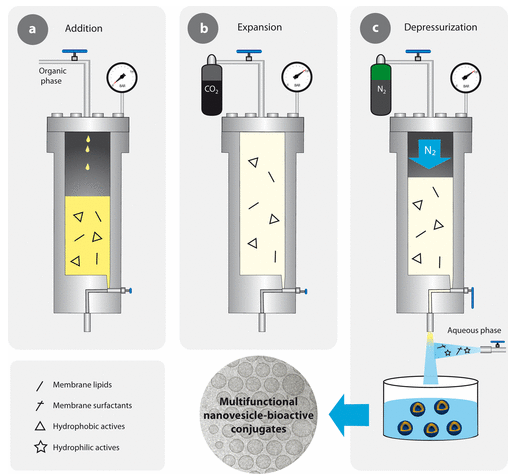
In this cutting-edge, we offer the possibility of preparing complexes composed of nanoparticles with or without molecules. The nanoparticles with antimicrobial properties can be prepared using the DELOS-SUSP method or other methods which can allow the preparation of nanoparticles while hydrophobic and hydrophilic drugs could also be encapsulated. A complete physicochemical characterization is then performed using different techniques such as DLS, ELS, MADLS, MLS, NTA, etc. This is followed by an evaluation of the antimicrobial performance of the complexes. In this step, the adhesion of microorganisms to surfaces under different conditions is studied. The formation of biofilms and microbial viability is also investigated.
Examples of CIBER BBN collaborative intramural projects implementing this Cutting-Edge Biomedical Solution:
Antimicrobial nanostructured biomaterials for complex wound healing (NABIHEAL), 101092269, European Union’s Horizon Europe research and innovation program, 01/01/2023-31/12/2026.
Services involved:
These studies are carried out by the U6 (Biomaterial Processing and Nanostructuring Unit) and the U16 (Surface Characterization and Calorimetry Unit). These two units are participating in a European project and they have a strong background of collaboration between them analyzing the antimicrobial properties of the nanoparticles loaded with and without drugs. Both units take part on the design, discussion, and evaluation of the nanoparticles or complexes. These nanoparticles with antimicrobial properties are prepared and physicochemically characterized by U6 and the evaluation of the antimicrobial activity of the complexes is studied in the U16.
 |
Our Biomaterial Processing and Nanostructuring Unit (U6) is located at the Instituto de Ciencia de Materiales de Barcelona (ICMAB-CSIC), in Barcelona, and under the coordination of Professor Jaume Veciana and Prof. Nora Ventosa, current directors of NANOMOL Group, which is a research group with wide expertise and recognized excellence in the synthesis, processing and study of molecular and polymeric materials with chemical, electronic, magnetic and biomedical properties. It gathers several laboratories, perfectly equipped, to perform the mission of this facility: the development, characterization, and large-scale production of molecular biomaterials of therapeutic or biomedical interest, with controlled micro-, nano- and supramolecular structure. One example of Key-Enabling-Technology (KET) available in this unit is a simple one-step methodology, DELOS-SUSP, based on the use of compressed fluids (CF), such as CO2, to prepare particulate materials with precise and reproducible structural characteristics at micro-, nano- and supramolecular levels (size, shape, internal structural gradients, supramolecular organization and crystalline purity). This example shows one of the singularities of this unit is that counts with CF–based plants at different scales, from mL to L, which allow process development by QbD and process scale-up.
The NANBIOSIS Unit 6 is works under the ISO9001 certification for standard quality control system
|
 |
The purpose of our Surface Characterization and Calorimetry Unit (U16) is to give support for the analysis of surface composition to researchers. This facility allows a broad knowledge about the chemistry of surfaces by the combination of the information of elements presents and their coordination state, given by the XPS technique, and the molecular structure of surface provided by the ToF-SIMs equipment. Its capabilities permit to work with extended and powdered solids, including those from biological origin as cells and bacteria. The characteristics of thin films on surfaces, as thickness, composition or electrical conductivity, can be also analyzed in this unit by spectroscopic ellipsometry. In addition, calorimetric analysis can be performed to test surface reactivity on powdered solids. ITC calorimetry facility is also under the Unit scheme.
Surface analysis laboratory fulfils the ISO9001:2008 (ES050823-1). |