Enhance the safety and efficacy of your biomedical device by evaluating its antimicrobial properties and behavior in realistic physiological environments.
Scientific Leader / leaders of the project: María Luisa González / Francisco M. Sánchez Margallo
Coordinator of the project: Margarita Hierro / Francisco M. Sánchez Margallo
Infections within the hospital environment and associated with biomedical devices are a significant public health concern. In this context, it is crucial to develop biomedical devices with antibacterial properties. This cutting-edge approach opens up the possibility of assessing the functionality of a biomedical device, such as implants, probes, medical and hospital equipment, in both small and large animal models, while simultaneously evaluating its antimicrobial activity in in vitro tests simulating the physiological environment bioreactors that simulate biofilm formation. Within this general objective, this service provides a broad understanding of the behaviour of implantable devices in the physiological fluid environment by characterising the surface of such devices. In addition, the evaluation of the antibacterial behaviour of biocompatible devices will be carried out by means of in vitro tests with bacteria, which can be performed in both static and dynamic conditions.
This service provides an added value by offering the opportunity to conduct studies using both large and small animal models in accordance with ISO standards. Additionally, it enables in vitro analysis of new device’s antimicrobial capacity by simulating the formation of biofilms, a critical factor in bacterial resistance and growth, and how the surface of biomedical devices could be altered after removal for full characterisation.
Examples of scientific publications implementing this Cutting-Edge Biomedical Solution:
Examples of projects implementing this Cutting-Edge Biomedical Solution:
Services involved:
These studies are carried out by the U16 (Surface Characterisation and Bacterial Colonization Unit) in collaboration with Jesús Usón Minimally Invasive Surgery Center (JUMISC). These two units participated in a European project and have co-analysed the antimicrobial properties of biomedical devices, in this case, orthopaedic implants. Firstly, U16 conducted the characterisation of the implant surface and its antimicrobial capacity in vitro, while JUMISC analysed the biocompatibility, stability and resistance of the implant in animal models, as well as its antimicrobial properties in vivo. Both units participated in the design, discussion and evaluation of the results of the characterisation of this device. The services involved were:
 |
The objective of the Unit 16 is to support researchers in the analysis of surface properties, as well as to test and characterise surfaces against bacterial colonisation, allowing a broad understanding of the chemical and physical properties of surfaces. The combination of the information on the elements present and their co-ordination state, given by the XPS technique, and the molecular structure of the surface provided by the ToF-SIMs equipment, provides the chemical characterisation of the upper layers of the surfaces. The physical characterisation of the surfaces deals with properties such as surface free energy, hydrophobicity, topography, electrical and mechanical properties of the surfaces and surface forces. For this purpose, the Unit 16 has experience in handling different instruments such as goniometer, optical profilometer, zeta potential analyser and AFM. Its capabilities allow it to work with extended and powdered solids, including those of biological origin such as cells and bacteria. Along with physical and chemical characterisation, the Unit 16 can provide testing services for microorganism adhesion, viability and biofilm formation on surfaces. |
 |
Our Unit 21 is equiped with experimental operating rooms with high technology and high field instrumentation to promote methodological and translational research class in Biomedicine, both minimally invasive surgery and new procedures and in preclinical development for technical evaluation, medical devices, biomaterials, etc. Surgical facilities allow the development and use of small and large animal models for research and experimental surgery in several longitudinal areas: cardiovascular diseases, liver and digestive diseases, respiratory and systemic diseases, endocrinology and urology, gynecological and reproductive diseases, pediatric diseases, orthopedics and traumatology, ophthalmology and experimental surgery in all organ systems have the infrastructure for transplant development, implants, prosthesis, biomaterials, etc. under high quality experimental conditions that allow surgery, clinical follow up and obtaining biological samples in these models. |
 |
The JUMISC Animal Housing Unit (NANBIOSIS Unit 22) provides services to companies and research groups in many preclinical trials. This unit advises designs and conducts preclinical validation on demand. It has qualified professionals to design, monitor and validate any type of biomedical study. In addition this unit has been certified by the Spanish Agency of Medicines and Health Products (AEMPS) for studies in “Dosing of test substance and non-clinical specimen drawing” and “Biocompatibility studies of medical devices”. These two new certifications are attached to existing “in vivo toxicity”, “Tolerance” and “Pharmacodynamics”. All these certifications allow NANBIOSIS members to perform studies to verify the effectiveness, safety and biocompatibility of nanotechnology under development. A most singular fact makes this unit is the possibility of housing, maintaining and supervising large animals for preclinical validations. This Service has extensive experience in handling and care of minipigs and what it entails use in preclinical studies. |
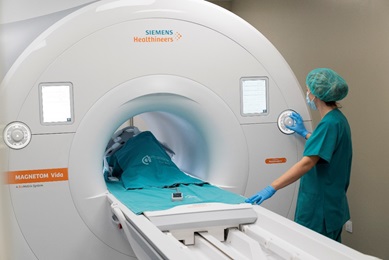 |
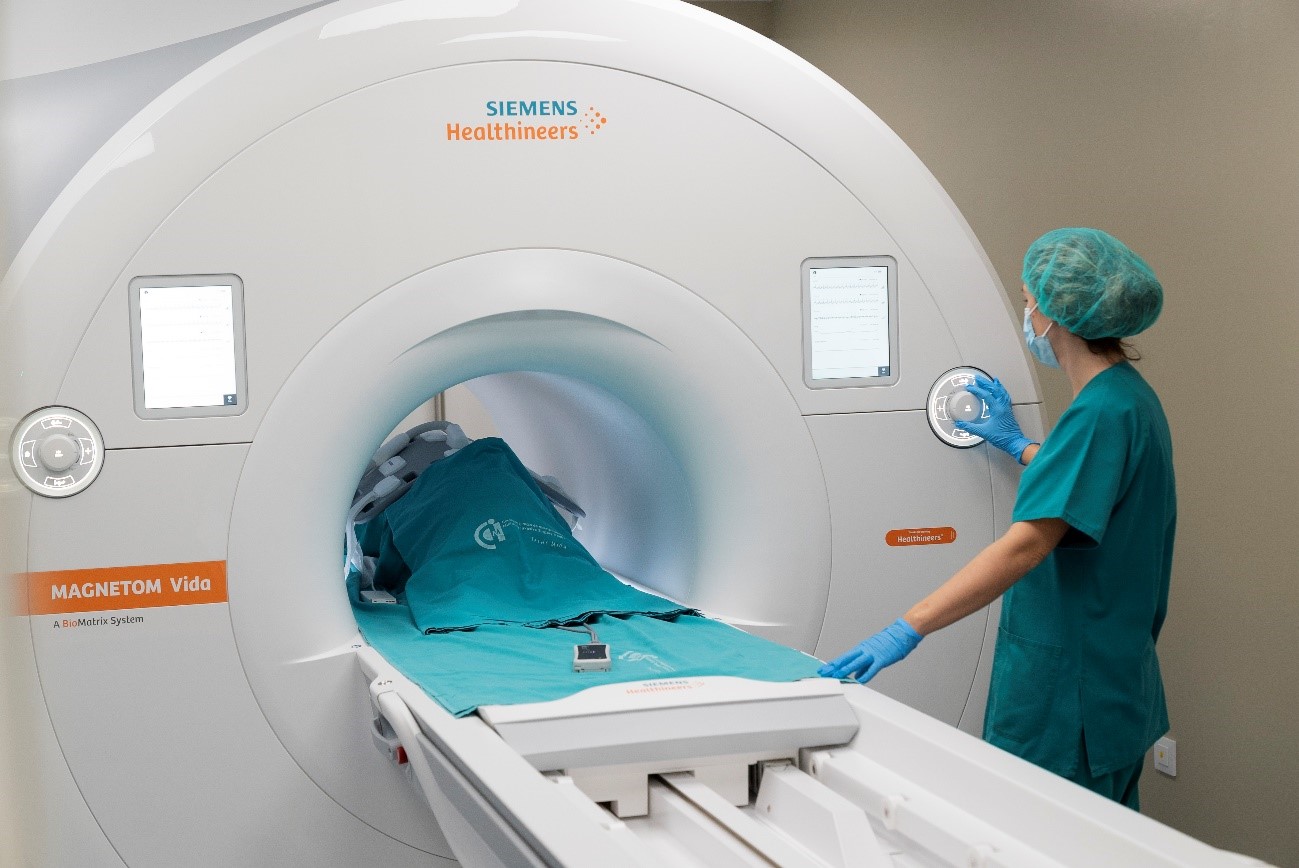
The range of medical imaging capabilities available within our Unit 24 allow for large animal diagnostic imaging, with the possibility to integrate all the information on body organs,systems, specific diseases or animal models, etc., that can be obtained with different imaging systems,such as clinical grade Magnetic Resonance Imaging, ultrasonography CT and fluoroscopy. This Unit is highly focused on cardiovascular diseases imaging and therapy, offering a comprehensive cardiac minimally invasive service ranging from disease modeling to therapy evaluation. Regulatory requirements prior to introducing a new medical diagnostic or therapeutic technology in clinical practice determine a mandatory preclinical validation in animal models; an scenario for which this Unit is especially suited. |