
NANBIOSIS Unit 10 develops lipid-based nanoparticles for gene and drug delivery, aiming for cost-effective, non-invasive treatments for retinitis pigmentosa.
Vitoria, february 2025. Retinitis pigmentosa (RP) is a congenital rare disease that leads to progressive and irreversible vision loss. Both genetic mutations and inflammation contribute significantly to the progression of the disease. Researchers from the Drug Formulation Unit (U10) of NANBIOSIS, based at the University of the Basque Country (UPV/EHU), are pioneering innovative strategies to combat this condition. Their work focuses on developing lipid-based nanovehicles for gene and drug delivery, aiming for cost-effective and non-invasive treatments for RP patients.
Gene Therapy: A Novel Approach to Address Retinal Mutations
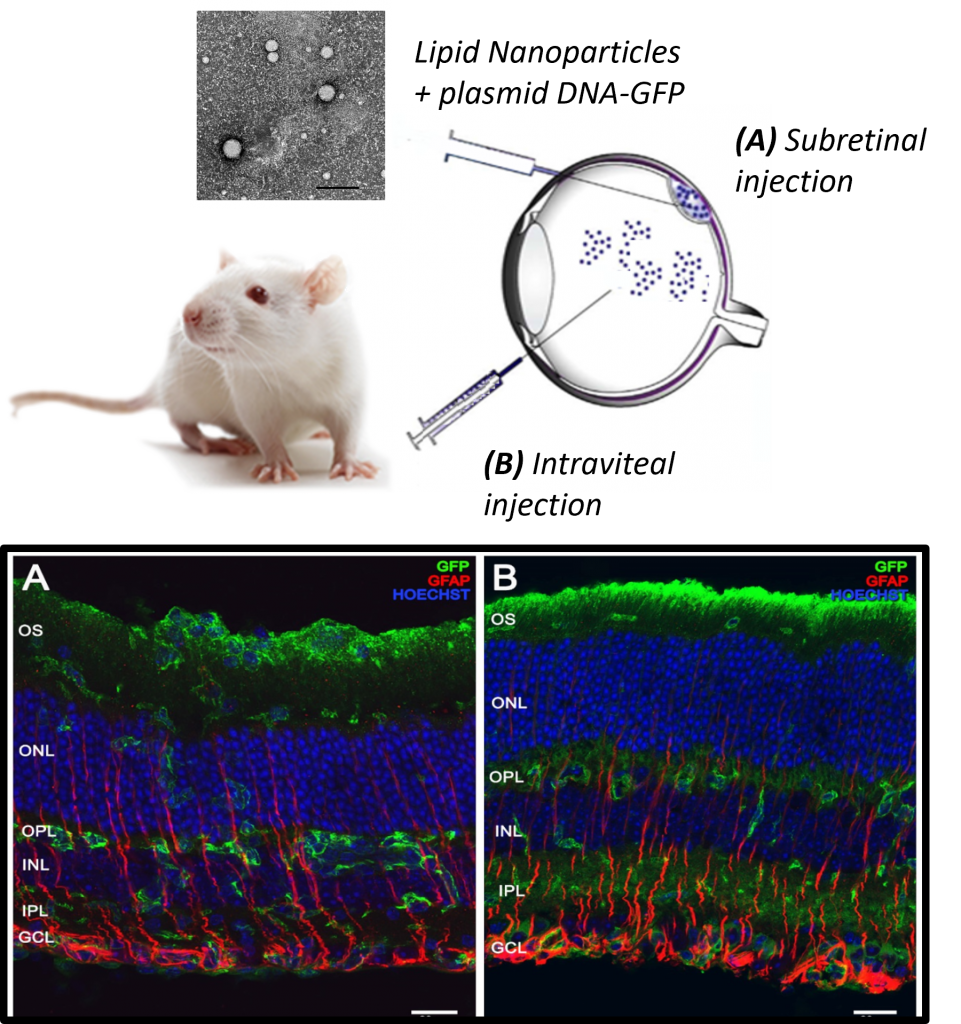
Unit 10 is engineering lipid-based nanoparticles to deliver genetic material to the retina effectively. In collaboration with the research group of Eduardo Fernández Jover, at Universidad Miguel Hernández (CIBER-BBN), they are testing these nanoparticles by delivering plasmids encoding the green fluorescent protein (GFP) to retinal layers. This strategy allows researchers to assess gene delivery efficiency at different retinal depths. Since over 200 gene mutations have been identified at the outer retinal layer that contribute to severe retinal diseases, reaching these layers is crucial for therapeutic success. The next phase of research involves incorporating therapeutic genes such as RPE65, one of the most frequently mutated genes in RP, to evaluate their potential clinical benefits.
Anti-Inflammatory Drug Delivery: Enhancing Retinal Protection
Alongside gene therapy, the team is exploring the use of lipid-based nanoparticles to deliver anti-inflammatory agents. In collaboration with the research group of Regina Rodrigo, at Centro de Investigación Príncipe Felipe (CIBER-ER), they aim to encapsulate therapeutic compounds in these nanocarriers, protecting them from degradation and enabling controlled release into the retina. Notably, they have achieved successful non-invasive administration via eye drops, culminating in the application for a PCT patent (PCT/EP2024/052232). The next steps involve expanding this technology to treat additional retinal inflammatory conditions and investigating the encapsulation of other anti-inflammatory compounds.
“We aim to face retinitis pigmentosa by a multidisciplinary strategy using lipid nanoparticles to target the retina as potential cost-effective and non-invasive treatments for patients.”
Idoia Gallego Garrido, Scientific Coordinator of Unit 10.
A Multidisciplinary and Translational Strategy
The combined gene and drug delivery approaches spearheaded by Unit 10 offer a promising outlook for RP patients. By leveraging lipid nanoparticles, the researchers aim to develop cost-effective and non-invasive therapeutic solutions.
This research aligns with the commitment of NANBIOSIS to bridging scientific innovation with translational medicine, fostering collaborations between academia and industry to accelerate the development of advanced therapeutic solutions.
References
- Olivares-González, L., Velasco, S., Gallego, I., Esteban-Medina, M., Puras, G., Loucera, C., Martínez-Romero, A., Peña-Chilet, M., Pedraz, J. L., & Rodrigo, R. (2022). An SPM-Enriched Marine Oil Supplement Shifted Microglia Polarization toward M2, Ameliorating Retinal Degeneration in rd10 Mice. Antioxidants (Basel, Switzerland), 12(1), 98. https://doi.org/10.3390/antiox12010098
- Al Qtaish, N. H., Villate-Beitia, I., Gallego, I., Martínez-Navarrete, G., Soto-Sánchez, C., Sainz-Ramos, M., Lopez-Mendez, T. B., Paredes, A. J., Javier Chichón, F., Zamarreño, N., Fernández, E., Puras, G., & Pedraz, J. L. (2023). Long-term biophysical stability of nanodiamonds combined with lipid nanocarriers for non-viral gene delivery to the retina. International Journal of Pharmaceutics, 639, 122968. https://doi.org/10.1016/j.ijpharm.2023.122968
What is NANBIOSIS?
The goal of NANBIOSIS is to provide comprehensive and integrated advanced solutions for companies and research institutions in biomedical applications. All of this is done through a single-entry point, involving the design and production of biomaterials, nanomaterials, and their nanoconjugates. This includes their characterization from physical-chemical, functional, toxicological, and biological perspectives (preclinical validation).
Leading scientists
The main value of NANBIOSIS is our highly qualified and experienced academic scientists, working in public institutions, renowned universities and other research institutes.
Custom solutions
Designed for either scientific collaboration or the private industry, we adapt our services to your needs, filling the gaps and paving the way towards the next breakthrough.

Cutting-Edge facilities
Publicly funded, with the most advanced equipment, offering a wide variety of services from synthesis of nanoparticles and medical devices, including up to preclinical trials.
Standards of quality
Our services have standards of quality required in the pharmaceutical, biotech and medtech sectors, from Good Practices to ISO certifications.
In order to access our Cutting-Edge Biomedical Solutions with priority access, enter our Competitive Call here.
NANBIOSIS has worked with pharmaceutical companies of all sizes in the areas of drug delivery, biomaterials and regenerative medicine. Here are a few of them:









