The Andalusian Center for Nanomedicine and Biotechnology (BIONAND) and the Center for Biomedical Research Network in Bioengineering, Biomaterials and Nanomedicine (CIBER-BBN), both partners of NANBIOSIS, in collaboration with the International Registry of Skeletal Dysplasias of the University of California (Los Angeles) and Masaryk University, of the Czech Republic have described a new genetic disease of the skeleton using a precision medicine strategy.
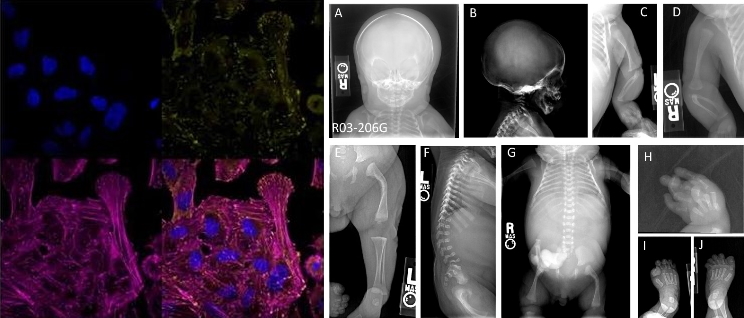
This disease consists of extreme bone fragility with lack of mineralization and skeletal deformation associated with joint dislocation and heart disease, as well as a lung deficiency that causes perinatal lethality -at the time of birth-. Using massive sequencing methods – of all genes – researchers have identified the mutations that caused a type of rare bone pathology, specifically, those of the ‘LAMA5’ gene, responsible for encoding a cellular matrix protein that surrounds blood vessels in skeletal tissues.
“Our scientific team has spent years investigating rare genetic syndromes that affect the skeleton in order to provide a medical solution to patients with difficult diagnosis and treatment,” explains the researcher from the Department of Cell Biology, Iván Durán, lead author of this study, whose results have been published in the scientific journal ‘EBIOMEDICiNE’.
According to the expert, precision medicine is the key to discovering what genetic and molecular factors cause this type of pathology and, therefore, understanding the mechanism that causes them and being able to develop personalized therapies.
Thus, researchers have also described the disease mechanism by generating cellular models by gene editing, mimicking the mutations in ‘LAMA5’, with the aim of confirming whether these are the origin and knowing the molecular process that triggers the problem. These cellular models have been generated by genetic editing with CRISPR, introducing mutations that cause a null or hypomorphic gene.
“Thanks to these models, we discovered a new signaling pathway that governs the formation of the skeleton – so that the bone grows and remains healthy – which means that our work has not only led to the discovery of a new disease, but to a mechanism unprecedented that can be exploited for common bone disorders ” –explains Durán, “the presence of ‘LAMA5’ between cells that direct skeletal formation indicates, therefore, that the appearance of signals from special blood vessels can be a very effective weapon for bone repair and regeneration. Blood vessels not only provide irrigation to the bone, but also carry signals and house niches of stem cells that can be mobilized to induce a regenerative process. ‘LAMA5’ seems to be a key component for harboring pericyte-type stem cells”.
Article of reference:
Barad M, Csukasi F, Kunova-Bosakova M, Martin J, Zhang W, Taylor SP, Dix P, Lachman R, Zieba J, Bamshad M, Nickerson D, Chong JX, Cohn DH, Krejci P, Krakow D, Duran I. Mutations in LAMA5 disrupts a skeletal noncanonical focal adhesion pathway and produces a distinct bent bone dysplasia. 2020 EBioMedicine. Nov 23;62:103075. doi: 10.1016/j.ebiom.2020.103075








