C. Franco, M. Mas-Torrent, A. Caballero, A. Espinosa, P. Molina, J. Veciana and C. Rovira
Chemistry A european Journal, 2015, 21, 5504 – 5509 DOI: 10.1002/chem.201405993
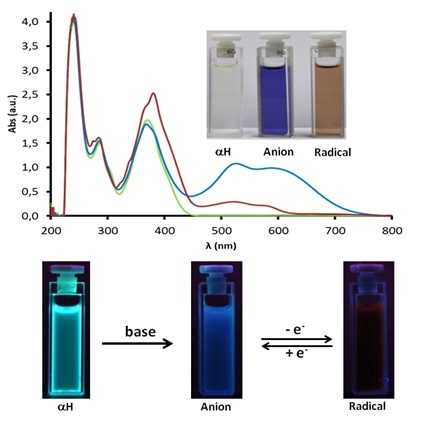
Two new pyrene-polychlorotriphenylmethyl (PTM) dyads and triads have been synthesized and characterized by optical, magnetic and electrochemical methods. The interplay between the different electronic states of the PTM moiety in the dyads and triads and the optical and magnetic properties of the molecules have been studied. The electronic spectra of the radicals show the intramolecular charge-transfer (ICT) transition at around 700 nm due to the acceptor character of the PTM radical. In the diamagnetic protonated derivatives, the fluorescence due to the pyrene is maintained, whereas in the radicals and the corresponding anions there is a clear quenching of the fluorescence. The redox activity of PTM radicals that are easily reduced to the corresponding carbanion has been exploited to fabricate electrochemical switches with optical and magnetic response.








