
Fabry disease therapy nanoGLA, developed by NANBIOSIS and our partners, shows superior efficacy in preclinical trials, targeting systemic and brain symptoms.
Barcelona, january 2025. An international research team led by the Institute of Materials Science of Barcelona (ICMAB-CSIC) and CIBER-BBN, in collaboration with the Institute of Advanced Chemistry of Catalonia (IQAC-CSIC), has developed a groundbreaking nanotechnology-based therapy called nanoGLA for the treatment of Fabry disease. The innovative solution has shown remarkable efficacy in preclinical studies and has been published in the open-access journal Science Advances (see below for reference links).
What is Fabry disease?
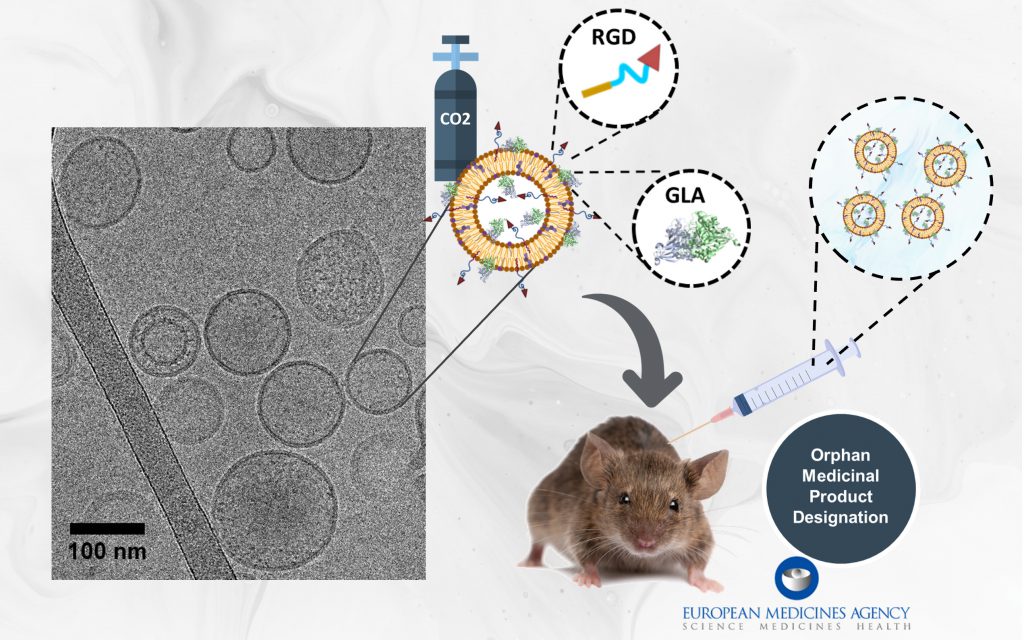
Fabry disease is a rare genetic disorder caused by a deficiency of the enzyme α-galactosidase A (GLA). This deficiency leads to the accumulation of fatty substrates (mainly globotriaosylceramide or Gb3) in cells, resulting in severe damage to various organs. The nanoGLA therapy employs peptide-guided nanoliposomes to deliver the deficient GLA enzyme effectively to the organs most affected by the disease. Researchers have successfully produced nanoGLA at the quality and scale required for preclinical trials, paving the way for clinical testing.
GLA inside nanoliposomes: nanoGLA
In studies with mouse models of Fabry disease, nanoGLA demonstrated superior efficacy compared to therapies using the non-encapsulated enzyme. It effectively targeted affected organs and, notably, the brain — a critical achievement that current therapies cannot match. These findings underscore nanoGLA’s potential to address both systemic and cerebrovascular manifestations of Fabry disease.
Highlighting the importance of this innovation, the European Medicines Agency (EMA) granted nanoGLA the Orphan Medicinal Product Designation in 2021, a significant milestone in its development.
The contribution of NANBIOSIS to this project was regarding the synthesis, processing and nanostructuring of the formulation (Unit 3 and Unit 6), and the preclinical assays using Fabry disease mouse models (Unit 20). Remember that if you want to collaborate with us, we are at the final stretch of our Open Call!
The product of scientific collaboration
This breakthrough is the result of collaborative efforts in which NANBIOSIS played a crucial role. These involve multiple international institutions, including ICMAB-CSIC, CIBER-BBN, Vall d’Hebron Research Institute (VHIR), and companies such as Nanomol Technologies SL and Leanbio SL, as well as IQAC-CSIC, the Institute of Biotechnology and Biomedicine (IBB-UAB), and international partners like Joanneum Research–Institute for Biomedical Research and Technologies (HEALTH) (Austria), Technion-Israel Institute of Technology (Israel), Guangdong-Technion Israel Institute of Technology (China), Aarhus University (Denmark), and Labcorp Drug Development (UK).
“The nanoGLA formulation represents a promising opportunity for Fabry disease patients, especially in addressing neurological symptoms, which current therapies fail to tackle.”
Elisabet González, ICMAB researcher and lead author
“The nanoGLA formulation represents a promising opportunity for Fabry disease patients, especially in addressing neurological symptoms, which current therapies fail to tackle,” said Elisabet González, researcher at ICMAB and one of the study’s lead authors. “Our goal is to develop safer and more effective treatments by harnessing the potential of nanotechnology.”
The bright future of this research
The research was conducted within the framework of the European project Smart4Fabry, funded by the European Union’s Horizon 2020 research and innovation program. Building on these promising results, the European Commission has provided further funding through the EU Phoenix and Nano4Rare projects to complete the preclinical phase and secure approval to begin clinical trials with human patients.
For more information, refer to the original study: “Targeted nanoliposomes to improve enzyme replacement therapy of Fabry disease,” published in Science Advances, Vol. 10, Issue 50, DOI: 10.1126/sciadv.adq4738.
This article was adapted from the press release by ICMAB. Press contact: communication@icmab.es. Institut de Ciència de Materials de Barcelona (ICMAB, CSIC).
What is NANBIOSIS?
The goal of NANBIOSIS is to provide comprehensive and integrated advanced solutions for companies and research institutions in biomedical applications. All of this is done through a single-entry point, involving the design and production of biomaterials, nanomaterials, and their nanoconjugates. This includes their characterization from physical-chemical, functional, toxicological, and biological perspectives (preclinical validation).
Leading scientists
The main value of NANBIOSIS is our highly qualified and experienced academic scientists, working in public institutions, renowned universities and other research institutes.
Custom solutions
Designed for either scientific collaboration or the private industry, we adapt our services to your needs, filling the gaps and paving the way towards the next breakthrough.

Cutting-Edge facilities
Publicly funded, with the most advanced equipment, offering a wide variety of services from synthesis of nanoparticles and medical devices, including up to preclinical trials.
Standards of quality
Our services have standards of quality required in the pharmaceutical, biotech and medtech sectors, from Good Practices to ISO certifications.
In order to access our Cutting-Edge Biomedical Solutions with priority access, enter our Competitive Call here.
NANBIOSIS has worked with pharmaceutical companies of all sizes in the areas of drug delivery, biomaterials and regenerative medicine. Here are a few of them:









