
Vasque Country, September, 2024 – In this interview, Lucía Enríquez, a PhD researcher at NANBIOSIS Unit 10, discusses her work on gene therapies for cystic fibrosis, a genetic disease that mainly affects the lungs. Her research focuses on using non-viral vectors to deliver gene-editing tools, like Prime Editing, a variation of CRISPR-Cas9, to correct mutations at the genetic level. Lucía explains the advantages of non-viral vectors, such as avoiding immune responses and offering safer, non-invasive treatment options. She also highlights the importance of interdisciplinary collaboration, particularly in her work at NANBIOSIS, where advanced drug formulation and pulmonary delivery systems are developed. Lucía reflects on the challenges of pursuing a scientific career in Spain, emphasizing the need for better working conditions and societal support for researchers.
Interviewer: Hi Lucía, tell us a bit about your research area. What projects are you currently working on?
Lucía: I’m currently working on my doctoral thesis in the laboratory of José Luis Pedraz, who is the director of Unit 10 of NANBIOSIS. My thesis focuses on the development of gene therapies encapsulated in non-viral vectors, mainly applied to the treatment of cystic fibrosis, a genetic disease with which our group has been collaborating for a long time. We work extensively on non-viral vectors, almost always applied to gene therapy, as well as other projects related to chemical molecules or other types of therapies.
Something very well-established in our group is that all of these treatments or developments must always be as patient-friendly as possible, meaning minimally invasive. In fact, one of the services we offer at NANBIOSIS is the development and characterization of pulmonary formulations. This is largely due to our experience with cystic fibrosis, as it’s a disease that often involves lung pathology, though it is not the only one.
In summary, we work on developing therapies that are non-invasive, often of genetic origin, applying the most cutting-edge and effective techniques possible.
Interviewer: Non-viral vectors, as I understand, differ from virus-based vectors in that they do not use the mechanisms of viruses. Additionally, they can act at various levels and don’t necessarily alter DNA. Can you tell us a bit more about these mechanisms and how they alter genes or their expression?
Lucía: There are many forms of gene therapy, as you mentioned. Among other types, some modify the genome sequence itself, while others alter the expression of that genome without modifying its sequence.
One of the most important aspects that many research groups focus on is the delivery of these gene therapy tools into the cell. The biggest challenge is ensuring that once inside a complex living organism, like a human, these tools reach the site where we need them to take effect. Historically, the most effective way to deliver these genetic tools was through viral vectors. These are modified forms of viruses that don’t cause the pathology typical of the virus but use the virus’s ability to infect a cell to deliver these genetic tools.
Non-viral vectors aim to achieve that delivery effectively and target the site where they need to act without using a viral vector. This avoids the negative aspects of viral vectors, such as immune responses, gene insertion in some cases, etc. However, non-viral vectors were very inefficient until the development of lipid nanoparticles, which is what the COVID vaccines are made of, where it became clear that this was a very clinically viable option.
Interviewer: No doubt that was a global boost, which had been in development for years, and had even been considered for cancer therapies, although outside clinical application. In your case, you say it’s for cystic fibrosis, which is a genetic disease as you mentioned. Do you work at the level of gene expression, at the gene level… what level do you edit at, and what tools do you use?
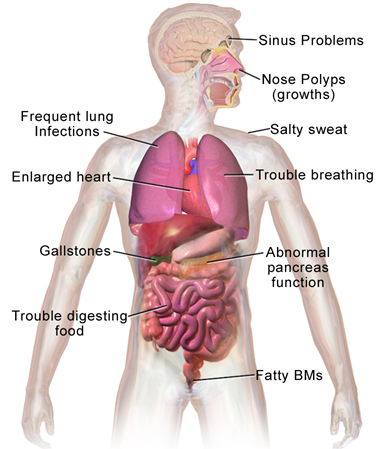
Lucía: Cystic fibrosis is a genetic disease, as I said, which can be caused by many different mutations. There’s one that is highly prevalent, accounting for over 40% of cases, which is a small deletion of three base pairs that causes issues with a chloride-transporting protein. This affects many organs in the body, but it’s especially important in the lungs because patients with cystic fibrosis accumulate a lot of mucus in their lungs and have serious breathing problems, as well as frequent respiratory infections, etc.
One approach to treating cystic fibrosis at the gene therapy level, which is being led by a colleague of mine who is also working on their thesis, involves delivering a healthy copy of the mutated gene in the form of a plasmid. This means it will promote the functional expression of the gene in a non-pathological way, but it won’t insert into the genome, and the expression won’t be permanent, so the treatment would need to be reapplied.
In my thesis project, we are developing genetic tools based on Prime Editing, which is a variation of the CRISPR-Cas system that corrects the mutation. These tools target the site where the three-base-pair deletion is located and correct it. Here, there is indeed an alteration of the patient’s genome sequence, with the goal of restoring a “wild-type” genotype, or a healthy sequence. This change in the sequence would be permanent in that cell and in all its daughter cells.
Interviewer: Right, when the cell divides, it preserves that gene through subsequent generations. Also, CRISPR has so many applications and is a hot topic. Can you briefly explain what the CRISPR-Cas technology consists of? How do you manage to edit such a specific gene so precisely?
Lucía: The CRISPR-Cas9 technology was discovered because it was originally a way bacteria could defend themselves from viruses. Essentially, it consists of two components: a protein called Cas9, which is a nuclease that cuts the double strand of the genome, and an RNA sequence that we call guide RNA.
The guide RNA scans the entire genome of the cell, and when it reaches a site where the base pairs match perfectly, the Cas9 protein binds to it, recognizes it, and cuts the double strand of DNA. This triggers many DNA repair mechanisms in the cell. If you only introduce the Cas9 protein and the guide RNA, what you usually create is a knock-out (a silenced gene). This happens because the cell tries to repair the sequence at all costs, but it often makes mistakes, like skipping base pairs or adding extra base pairs, in a desperate attempt to avoid cell death.
If, at the time you make the double-strand cut, you also introduce a DNA sequence that matches the genome sequence, there’s a chance the cell will incorporate that sequence as it attempts to repair the break. If you’re introducing a healthy sequence, you’re effectively curing the cell of the genetic disease it had.
What we do isn’t exactly CRISPR-Cas9. We use Prime Editing, which is a variation of this system where the protein doesn’t cut both strands of the DNA, only one of them. This allows you to introduce small insertions, deletions, or base pair changes. In our case, it’s useful because, as I mentioned, one of the most prevalent mutations in cystic fibrosis is a deletion of three base pairs. So, it’s simpler and more efficient in terms of genetic correction to insert those three base pairs using Prime Editing, which is still a variation of CRISPR, rather than introducing an entire genomic sequence to try to repair the gene.
Interviewer: It really is amazing. And this is a system that can be universalized for many different applications, not just for cystic fibrosis. There are so many different genetic diseases, and here you have a tool that you can simply adapt, I imagine, by changing the guide RNA and the sequence you want to introduce. This way, it could be applied to a completely different disease, right?
Lucía: Exactly. In fact, since it was discovered, this tool has been used by many research groups around the world for all kinds of genetic diseases.
Interviewer: Great. Let’s talk a bit about you and your scientific career. On a personal level, what motivated you to choose a career in science? You’re doing a PhD now—what made you think, “This is for me”?
Lucía: It was mainly curiosity. I’ve always considered myself a very curious person, constantly seeking to understand the reasons behind things. In the end, research is about pushing the boundaries of knowledge to go a bit further and see beyond what’s known. That fascinates me on every level, but it also fulfills me personally, because of the kind of person I am—someone who needs to know things, search for answers, solve problems. I think that’s something really cool.
Interviewer: Yes, and it’s something quite common in the scientific world. Many people get into it driven by that initial curiosity, asking, “Why is this like that? Why does it work this way?” Your scientific career has started recently—have you had any “Eureka” moments? Moments where you felt proud of something working out, or something you consider an achievement, either personally or professionally?
Lucía: Well, honestly, I think “Eureka” moments don’t happen that often. If you do have one of those moments, maybe you’ll win a Nobel Prize afterward (laughs). But I think it’s more about the day-to-day—the small achievements, the little things. It also depends a lot on what kind of research you do. If you’re in more basic research, where you’re trying to understand how things work or molecular processes, I think it’s easier to get one of those “Eureka” moments—like discovering the function of a specific protein or the implications of a certain process, etc.
For us, since we do more process development and optimization, unfortunately, 80, maybe even 90% of the results are negative (laughs). I think it’s more about the small wins, taking one step at a time, building little by little, rather than having a big “Eureka” moment.
Interviewer: And constantly hitting a wall, saying “It’s not working, it’s not working…” and then one day suddenly saying, “I did it, I know what went wrong!” Even in that 10-20% of success, it’s very satisfying, right?
Lucía: When it works, it’s very satisfying (laughs).
Interviewer: What advice would you give to young people considering a career like yours in science?
Lucía: When people ask me, I always tell them to explore a lot and talk to people. There’s no wrong path—you can go into research or not. There are many ways to stay connected to science without working in a lab. I think everyone has to find their own path. It’s a beautiful path—I enjoy it, and as I said before, being constantly at the edge of knowledge is very satisfying. But it also demands a level of dedication and sacrifice that not everyone may want in their life. And that’s fine too—it doesn’t make you any less valid if you don’t want this type of life. Plus, that doesn’t mean you can’t stay connected to science. So, talk to people, explore options—there are plenty out there. And that’s it (laughs).
Interviewer: And what do you think have been your biggest challenges in the field of scientific research?
Lucía: I think there have been too many. Everything is a challenge, and if it weren’t, we’d be doing something else, I think. I don’t know, I think when you’re dedicating yourself to learning, literally. There’s a point in the learning phase where you have to understand what’s happening. And that’s always a challenge when there’s no information in that field because, literally, you’re creating it yourself. It’s complex.
Interviewer: And it’s scary—you’re looking into the unknown, it’s the uncertainty, right? You have to enjoy that. How do you think we can encourage scientific vocations among young people?
Lucía: I think promoting a scientific career or this type of life comes down to making it accessible and providing good working conditions. While things have improved, they still aren’t good. Many people are still doing their PhD without getting paid because they don’t have access to a scholarship or funding source. That’s unacceptable. It’s very hard to achieve stability, a long-term career outlook, or a professional life that’s compatible with a personal life. I think that’s challenging, and I believe it’s the responsibility of authorities to promote it.
I also think there’s a societal issue. In Spain, society doesn’t see research as something necessary or even as a real job. I still get asked by people on the street or friends of my parents, “When are you going to stop doing that little course you’re taking?” And I’m like, “Wait, I’m not taking a course!” (laughs). I work in research—this is my job. It hasn’t really sunk in socially. So, if we don’t value it socially, it won’t be valued politically either, and then there won’t be funding. Sure, there are many calls for projects, etc., but the people working in this field need to be able to live, not just survive. They need to live under decent conditions. I think this is what most discourages people from pursuing a scientific career here in Spain, because it’s almost unfeasible.
Interviewer: The issue of job stability is an ongoing battle.
Lucía: To give you an idea, out of my group of friends from university who are doing research, five of us are working on our PhDs. I’m the only one doing it in Spain. Two are in Germany, and two are in the United States. Naturally, they all have a much better quality of life than I do.
Interviewer: It’s interesting because when data comes out about which professions people trust the most, scientists are at the top, even on par with doctors. People trust what scientists say. But as you pointed out, there’s a lack of societal awareness that science requires funding, public investment, and future prospects so that people who want to pursue it can have a stable career and decent working conditions. And many people who go abroad never come back because they’re treated so much better there…
Lucía: I spent 6 months working in a lab in Philadelphia, and the way they treat you—not just in terms of working conditions but also salary and work environment—is important. But what’s really key is the social recognition. Right now, to do a PhD, you need a degree, a master’s, and I’m earning almost the minimum wage, you know? We just want knowledge and qualifications to be valued in a rational way.
Interviewer: It’s not too much to ask…
Lucía: No (laughs). And I think this is important. It frustrates me that this is the reason why many people don’t go into science. There are so many brilliant and passionate people, but they eventually want to buy a house or start a family. And like this, you just can’t. And that’s the reality.
Interviewer: Let’s talk about NANBIOSIS. You work in Unit 10. Can you tell us a bit about what this unit focuses on, your role in the network, and your connection with it?
Lucía: Unit 10 is the Drug Formulation Unit. Essentially, it focuses on the development, characterization, and optimization of delivery systems for active ingredients, which could include chemical molecules, antibodies, proteins, gene therapy, and more. In short, we develop formulations that allow for the efficient delivery of these active ingredients.
This involves developing the optimal non-viral vector for each molecule, the composition of that non-viral vector, its formulation, and characterization. Additionally, we also focus on the characterization of pulmonary formulations, which is another key function of Unit 10. I believe we are a pretty advanced unit because we have pulmonary formulation characterization equipment, which is rare in Spain—there’s maybe only one other place with similar equipment.
Going back to non-invasive therapies, I think the pulmonary route is a very viable option, and it also allows us to characterize formulations intended for ophthalmic or intranasal delivery. Within this context, my role involves conducting experiments and designing them with the groups or entities that contact us to use our services or develop a project.
Interviewer: And connecting to this, how do you think NANBIOSIS can positively contribute to scientific research in the academic world?
Lucía: To be honest, before joining this lab, I didn’t know what NANBIOSIS was. When I discovered it, I thought it was a fantastic opportunity to create networks, collaborate, and connect with people, groups, and entities working on things different from your own. It’s also an ideal way to facilitate knowledge exchange between academia and industry, which I think is very important. Above all, it helps expand your mind and allows you to use your expertise to contribute to the development of others’ knowledge.
Interviewer: I imagine you’re referring to, for example, a company that needs to test a type of formulation or is looking to vectorize a drug or treatment. You provide all that support in terms of know-how, especially considering your lab is cutting-edge, with top-notch equipment and excellent academics. You have true experts in pharmacology, and a company can really benefit from that help.
Lucía: Absolutely. In fact, I think the private industry has the ability to bring the knowledge generated to the market—something that academia doesn’t have the capacity to do, due to the nature of how academia works.
In academia, knowledge is generated, and the industry has the capability to bring it to the market. But there needs to be a common ground between academia and industry for that process to happen. One of the things I like about NANBIOSIS is that it presents itself as a potential point where that connection can happen, and that’s great. As you mentioned, there are a lot of prestigious people in academia. Just to give an example, our principal investigator (PI), José Luis Pedraz, is a member of the Spanish Academy of Pharmacy.
Interviewer: In fact, José Luis Pedraz is “Académico de Número”, a Full Member of the Spanish Academy of Pharmacy—one of the top 50 pharmacists in the country!
Lucía: Absolutely. When it comes to developing formulations or understanding pharmacology processes, honestly, there are few people better in this country than José Luis Pedraz. Having the opportunity, through NANBIOSIS, to have a meeting point with industry to launch that knowledge and enhance that know-how, as you mentioned, is truly a fantastic opportunity.

Interviewer: Great. And how has NANBIOSIS contributed to your scientific career? I understand you work with the services NANBIOSIS offers and are developing your research—what has it provided you with professionally?
Lucía: As I mentioned, NANBIOSIS is a meeting point for different groups and entities, and it has given me the opportunity to connect and understand how people working in different research fields think. This is crucial if you’re in science. Progressing in science without interacting with other areas is almost like failing in the attempt, and José Luis understands this very well: you need to collaborate and understand all the fields developing around you.
In fact, it was one of the reasons I chose to do my PhD here—because of the culture of collaboration and working with other groups. We work with a couple of groups that are engineers specializing in developing materials and devices for medical applications. This is something you don’t initially consider, but when you have your formulation with your gene therapy all ready to go, you might then ask, “How do I administer it?” Having the opportunity to talk to people who develop delivery devices or understand that part of the process that you might not cover—because we can’t do everything—is vital for your development as a scientist and for understanding everything happening around you.
Interviewer: In addition, NANBIOSIS has a wide range of Units and a very broad, multidisciplinary service portfolio, which is absolutely essential in research and technology transfer today. In fact, this leads nicely into the next question: At NANBIOSIS, we have the Cutting Edge Biomedical Solutions, which involve combining several services from various Units to address a market problem or an industrial challenge. This aims to provide solutions to the industry on issues that require that know-how and the interconnection and synergy between the Units. You have been involved in several of these Cutting Edge Biomedical Solutions; could you give us a brief overview of them?
Lucía: Yes, currently we have three active ones, if I remember correctly. They all revolve around nanomedicines and non-viral vectors, encapsulating active ingredients, cells, genetic material, proteins, etc.
One is focused on the physicochemical characterization of these nanomedicines themselves. Having them well-characterized and studied allows us to understand exactly what’s happening and makes the scaling process easier.
Another one is about in vitro characterization of these nanomedicines. This means studying how they behave in pathological models or cellular models in two dimensions. This allows you to start fine-tuning the formulations or nanomedicines to ensure they have biological activity.
The third one is about in vivo characterization of these medicines. This helps you understand how these nanomedicines work within a more complex organism compared to a two-dimensional cell culture. Using experimental animals, you can study how they distribute within the organism, how effective they are, etc. This enables a better understanding of how the therapy works and optimizes it in a complex organism before moving on to human clinical trials.
All three together cover the necessary steps before reaching clinical trials.
Interviewer: They are essential in the transfer and translation of new therapies, and require a lot of hands-on work and cutting-edge facilities. One last question: How do you see yourself in 5 or 10 years?
Lucía: To start with, I hope to be a doctor (laughs).
Interviewer: How long have you been working on your thesis?
Lucía: Well, it’s been about two and a half years now, so we’re about halfway through. And then… I don’t know. Science is something that I really like and motivates me a lot, and it’s always been part of my life’s project. I think this happens not only to people in science: if you dedicate yourself to something that motivates you a lot and you’re willing to give it 100% every day, it becomes part of your life’s project, not just your job. But it’s not my only life project (laughs), so… we’ll see. We’ll see what opportunities arise, whether I can continue dedicating myself to science or if it stops being viable. As I mentioned before, it’s not an easy path; I might be able to pursue science… but maybe not in this country. I don’t know, we’ll see.
Interviewer: We’ll see. Thank you very much for these minutes. It’s been a pleasure, Lucía. We’ll stay in touch.
Lucía: Likewise, see you later!
You can watch the full interview here (Spanish):
What is NANBIOSIS?
The goal of NANBIOSIS is to provide comprehensive and integrated advanced solutions for companies and research institutions in biomedical applications. All of this is done through a single-entry point, involving the design and production of biomaterials, nanomaterials, and their nanoconjugates. This includes their characterization from physical-chemical, functional, toxicological, and biological perspectives (preclinical validation).
Leading scientists
The main value of NANBIOSIS is our highly qualified and experienced academic scientists, working in public institutions, renowned universities and other research institutes.
Custom solutions
Designed for either scientific collaboration or the private industry, we adapt our services to your needs, filling the gaps and paving the way towards the next breakthrough.

Cutting-Edge facilities
Publicly funded, with the most advanced equipment, offering a wide variety of services from synthesis of nanoparticles and medical devices, including up to preclinical trials.
Standards of quality
Our services have standards of quality required in the pharmaceutical, biotech and medtech sectors, from Good Practices to ISO certifications.
In order to access our Cutting-Edge Biomedical Solutions with priority access, enter our Competitive Call here.
NANBIOSIS has worked with pharmaceutical companies of all sizes in the areas of drug delivery, biomaterials and regenerative medicine. Here are a few of them:









