The Soft Lab held a new edition of the course on characterization and preparation of particulate materials
José Amable Bernabé, Technical Coordinator of Unit 6, hosted a course on “Characterization techniques for particulate materials”.
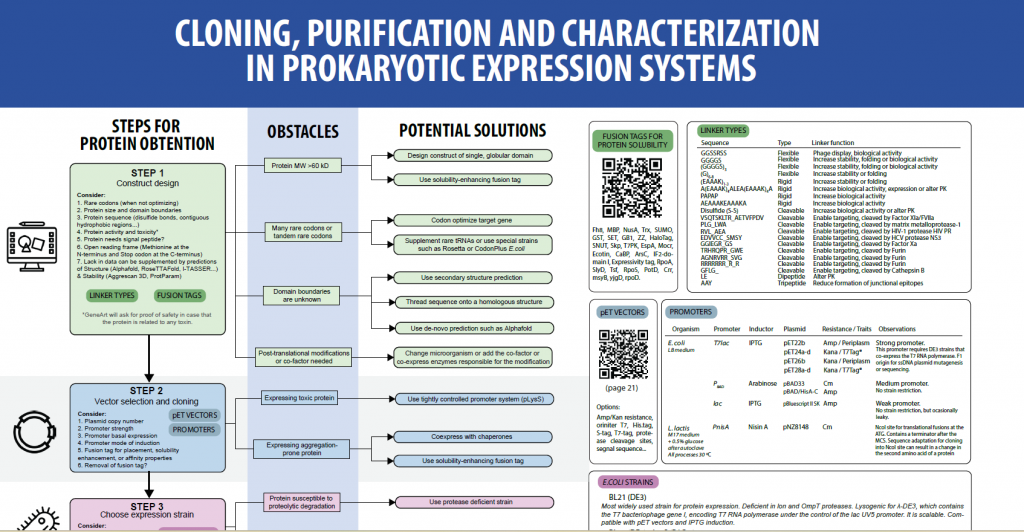
Barcelona, october 2024. For yet another year, José Amable Bernabé, of the Soft Materials Service at ICMAB and Technical Coordinator of NANBIOSIS Unit 6, offered this course. The course was an explanation of different techniques to characterize nanoparticles and particulate matter, including the fundamentals of these techniques, sample preparation, practical examples and results interpretation.
The course is offered every year through the CSIC training courses offered every year for all its staff, as reported in ICMAB webpage.
Soft Materials Service
The Soft Materials Service provides equipment and technical assistance for the preparation and characterization of micro-and nanostructured soft molecular materials (molecular surfaces, micro- and nanoparticulate molecular materials, plastic films, dispersed systems, SAMs, etc..) with interest in different areas of application (biomedicine, electronics, energy storage and other chemical and material application areas).
The Soft Materials Service, with Amable Bernabé and David Piña as technicians, participate in many European projects and give service to the whole ICMAB community, apart from the Nanomol Research Unit, and also to other CSIC centers and research institutions and companies.
On April 2022, the Service Materials Service, which is part of our Unit 6, obtained the ISO 9001:2015 Quality Certification, which ensures the quality of the service provided and helps to continue with its improvement and extension to future services.

What is NANBIOSIS?
The goal of NANBIOSIS is to provide comprehensive and integrated advanced solutions for companies and research institutions in biomedical applications. All of this is done through a single-entry point, involving the design and production of biomaterials, nanomaterials, and their nanoconjugates. This includes their characterization from physical-chemical, functional, toxicological, and biological perspectives (preclinical validation).
In order to access our Cutting-Edge Biomedical Solutions with priority access, enter our Competitive Call here.
NANBIOSIS has worked with pharmaceutical companies of all sizes in the areas of drug delivery, biomaterials and regenerative medicine. Here are a few of them: