Innovative Copper-Based Nanoparticles Open New Frontiers in Glioblastoma Treatment
NANBIOSIS Unit 9 advances glioblastoma treatment with tailored copper nanoparticles, reducing tumor invasiveness and opening new therapeutic pathways.
Zaragoza, December 2024. Our Unit 9, specialized in nanoparticle synthesis, has achieved a breakthrough in the fight against glioblastoma, one of the most aggressive and treatment-resistant forms of cancer. Utilizing state-of-the-art facilities and expertise, the Unit and its collaborators have developed novel copper-based nanostructures with tailored release patterns, demonstrating significant potential in inhibiting tumor progression and invasiveness.
A New Paradigm in Cancer Therapy
Glioblastoma (GBM) is characterized by its high invasiveness and poor prognosis. Current therapeutic options are limited by the tumor’s ability to infiltrate surrounding tissues, making complete surgical removal and effective treatment challenging. Unit 9 has addressed these challenges by synthesizing copper-based nanoparticles with controlled release kinetics, a cutting-edge approach that enhances localized therapy while minimizing systemic toxicity.
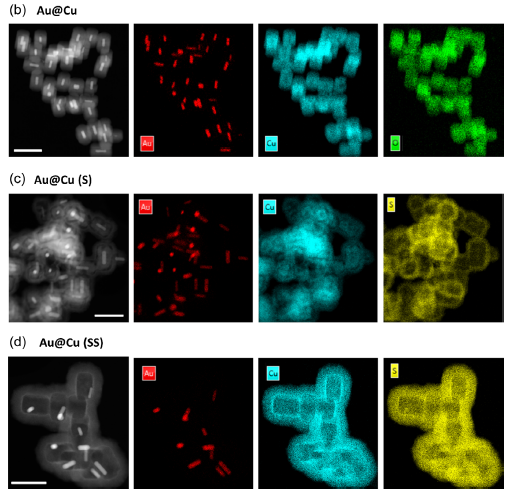
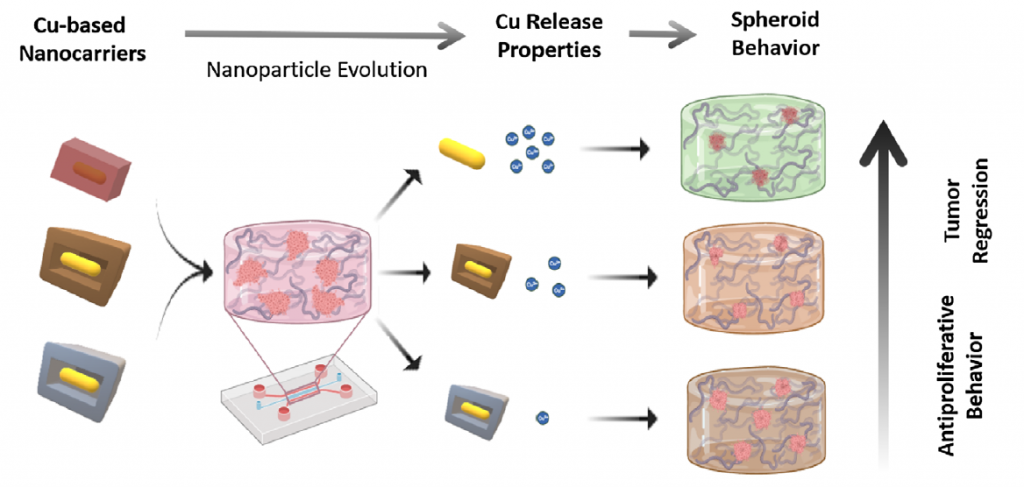
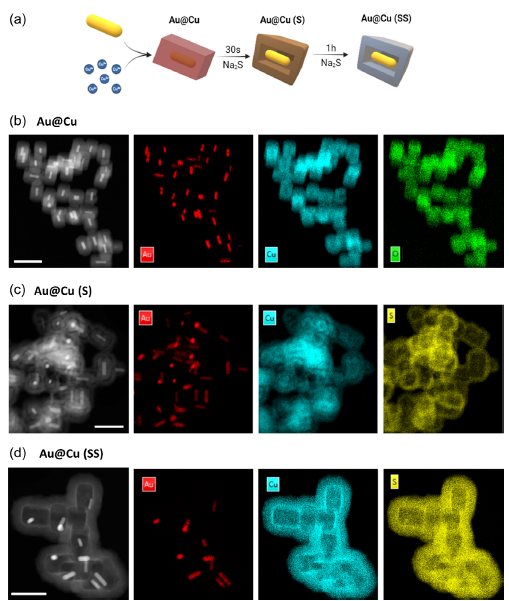
Through a meticulous synthesis process, the authors of the publication created a series of nanoparticles, including Cu2O and core-shell configurations, which were further enhanced by controlled sulfidation techniques. These advancements allow precise tuning of copper ion release, adapting to the tumor microenvironment and directly influencing tumor proliferation and invasiveness.
Translating Innovation into Impact
In collaboration with leading academic and industrial partners, the efficacy of these nanoparticles was evaluated in advanced 3D tumor models. These models, developed using microfluidic devices, replicate the complex architecture of glioblastoma tumors, enabling realistic assessments of therapeutic outcomes. Results revealed that copper release intensity strongly correlates with a reduction in tumor spheroid size, invasiveness, and malignancy markers.
Key findings include:
- Enhanced Targeting: High-precision release patterns disrupted glioblastoma cell proliferation and inhibited the formation of invasive protrusions.
- Reduced Aggressiveness: Nanoparticles shifted the tumor phenotype to a less invasive state, as evidenced by changes in epithelial-to-mesenchymal transition markers.
- Scalability and Customization: The synthesis protocol offers scalability and adaptability for diverse therapeutic needs.
Opportunities for Collaboration
These advancements underscore the transformative potential of nanoparticle-based therapies in oncology. From NANBIOSIS, we would like to extend an invitation to pharmaceutical companies and research institutions to explore collaborative opportunities within our Units. By integrating these technologies into drug development pipelines, stakeholders can accelerate the transition from laboratory research to clinical application, addressing critical unmet needs in glioblastoma treatment.
About NANBIOSIS Unit 9
Our Unit 9 specializes in the synthesis of high-quality nanoparticles, offering tailored solutions for biomedical applications. As part of the NANBIOSIS-ICTS, the Unit combines cutting-edge technology and expertise to drive innovation in nanomedicine, supporting both academic and industrial R&D initiatives.
For more information and to discuss potential partnerships, visit the porfolio of Unit 9 here.
What is NANBIOSIS?
The goal of NANBIOSIS is to provide comprehensive and integrated advanced solutions for companies and research institutions in biomedical applications. All of this is done through a single-entry point, involving the design and production of biomaterials, nanomaterials, and their nanoconjugates. This includes their characterization from physical-chemical, functional, toxicological, and biological perspectives (preclinical validation).
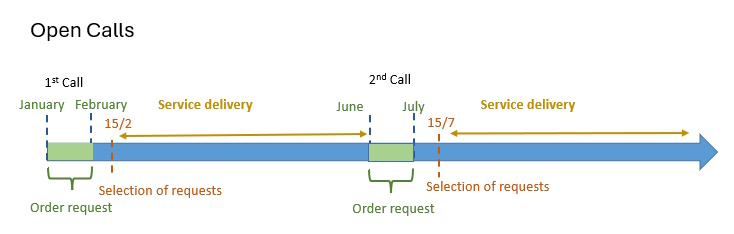
In order to access our Cutting-Edge Biomedical Solutions with priority access, enter our Competitive Call here.
NANBIOSIS has worked with pharmaceutical companies of all sizes in the areas of drug delivery, biomaterials and regenerative medicine. Here are a few of them: