Release of targeted protein nanoparticles from functional bacterial amyloids: A death star-like approach
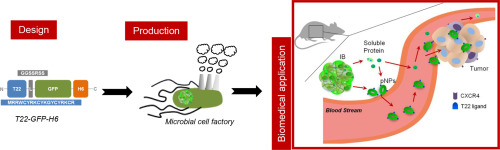
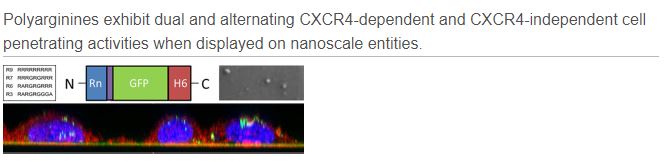
Sustained release of drug delivery systems (DDS) has the capacity to increase cancer treatment efficiency in terms of drug dosage reduction and subsequent decrease of deleterious side effects. In this regard, many biomaterials are being investigated but none offers morphometric and functional plasticity and versatility comparable to protein-based nanoparticles (pNPs). Researchers of NANBIOSIS units 1 and 18 are co-authors of an article publish by Journal of Controlled Release in which it is described a new DDS by which pNPs are fabricated as bacterial inclusion bodies (IB), that can be easily isolated, subcutaneously injected and used as reservoirs for the sustained release of targeted pNPs. Our approach combines the high performance of pNP, regarding specific cell targeting and biodistribution with the IB supramolecular organization, stability and cost effectiveness. This renders a platform able to provide a sustained source of CXCR4-targeted pNPs that selectively accumulate in tumor cells in a CXCR4+ colorectal cancer xenograft model. In addition, the proposed system could be potentially adapted to any other protein construct offering a plethora of possible new therapeutic applications in nanomedicine.
In the study the researchers have generated novel smart biomaterials gathering most of the desirable features for implantable DDS, with cost effectiveness and simplicity in the biofabrication process. In this regard, single step fabricated IBs when injected subcutaneously rendered a long lasting release of targeted pNPs, able to enter to the blood stream and specifically target the tumor for as long as 10 days and they have described for the first time an approach for the fabrication of protein DDS based on protein deposition as IBs and their sustained release in form of fully functional targeted pNPs. This technology provides and stable source of targeted protein nanoparticles during long periods within the body with the action at distal points from the implantation site and pave the way for the appearance of new more efficient and less invasive treatments for a broad number of pathologies.
Protein production has been partially performed by the ICTS “NANBIOSIS”, more specifically by the U1. Protein Production Platform (PPP), whereas the in vivo biodistribution assays were performed in the NANBIOSIS U18. Nanotoxicology Unit,
For further information see https://sciencedirect.com/science/article/pii/S0168365918301780?via%3Dihub