U6-S01. Use of High-pressure laboratory-scale plant
Use of High-pressure laboratory-scale plant
with 50, 100 and 300 mL reactors for the processing of biomaterials. Processing of cytotoxic compounds when required.
Preparation of soft molecular materials with controlled structure at supramolecular, micro- and nanoscopic level, using one-step methodologies based on green compressed fluids (i.e. compressed and supercritical CO2).Micro- and nanoparticulate single compounds with high supramolecular homogeneity (i.e., pure polymorphic phases, materials with single polymer folding, etc.).
- Particulate polymeric matrix uniformly loaded with active compounds (therapeutics, cosmetic ingredients, catalyst, pigments, and dyes, etc.)
- Dispersed systems (suspensions, liposomes, emulsions, vesicles) with narrow particle size distribution and high morphological homogeneity.
- Porous materials, either crystalline or amorphous, with defined porosity and porous size.
Customer benefits
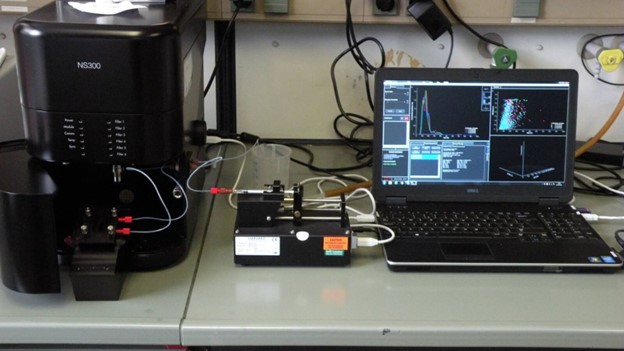
Lab-scale high pressure systems, based on a 50 mL, a 100 mL and a 300 mL stirred high pressure autoclaves equipped with pumps for the supply of compressed fluids and liquid solutions. The high-pressure systems can also optionally be equipped with several filters, manometers, thermocouples, and back pressure regulators. The maximum operative pressure is 23 MPa and the maximum operative temperature is 200 °C.
The 300 mL system is also equipped with a mass flowmeter, and a data acquisition system.
All the plants have been designed for micro- and nanostructuring molecular and soft materials.
Target customer
- Pharmaceutical industry
- Food Industry
- Chemical industry
- Materials research centers
References
- M.Köber, et al., J.C.I.S 2023, 631, 202-211.
- E.Elizondo,et al., J. Am. Chem.Soc. 2012, 134, 1918−192.
- I.Cabrera, et al. Nano Lett. 2013, 13, 3766−3774.